Marine phasolosma esculenta fibrinolytic enzyme, preparation method and applications thereof
A kind of Delicious Cosmia and plasmin technology, applied in the application of stroke and anti-tumor, treatment of thrombosis, the field of preparation of marine Delicious Cosmia plasmin, can solve problems such as unsatisfactory
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] 《Example 1》Preparation of Plasmina plasmolytic enzyme from the sea
[0040] Experimental Materials : Phascolosoma esculenta (Phascolosoma esculenta), purchased from Anhai City, Jinjiang, Fujian Province.
[0041] preliminary separation : Put the fresh and delicious Dermocystus in the seawater pool for a few days, and excrete the visceral impurities. According to the results of the active site detection, take the yellow intestinal tissue, add a certain volume of 0.02mol / L Tris-HCl buffer solution (pH7.4), cut it with scissors, put it in the refrigerator, and store it between -20°C and 4°C Repeated freezing and thawing 3 times. Put the tissue into the homogenizer for a few seconds, take it out and centrifuge at 10,000 rpm for 10 min at 4°C to remove the precipitate. Measure the volume of the supernatant and mix it with saturated ammonium sulfate of pH 7.4 to make the concentration of ammonium sulfate in the solution reach 30%, place at 4°C for 12h. Then refrigerate...
Embodiment 2
[0051] "Example 2" measures the method for the activity of Plasmodium pallidum fibrinolytic enzyme:
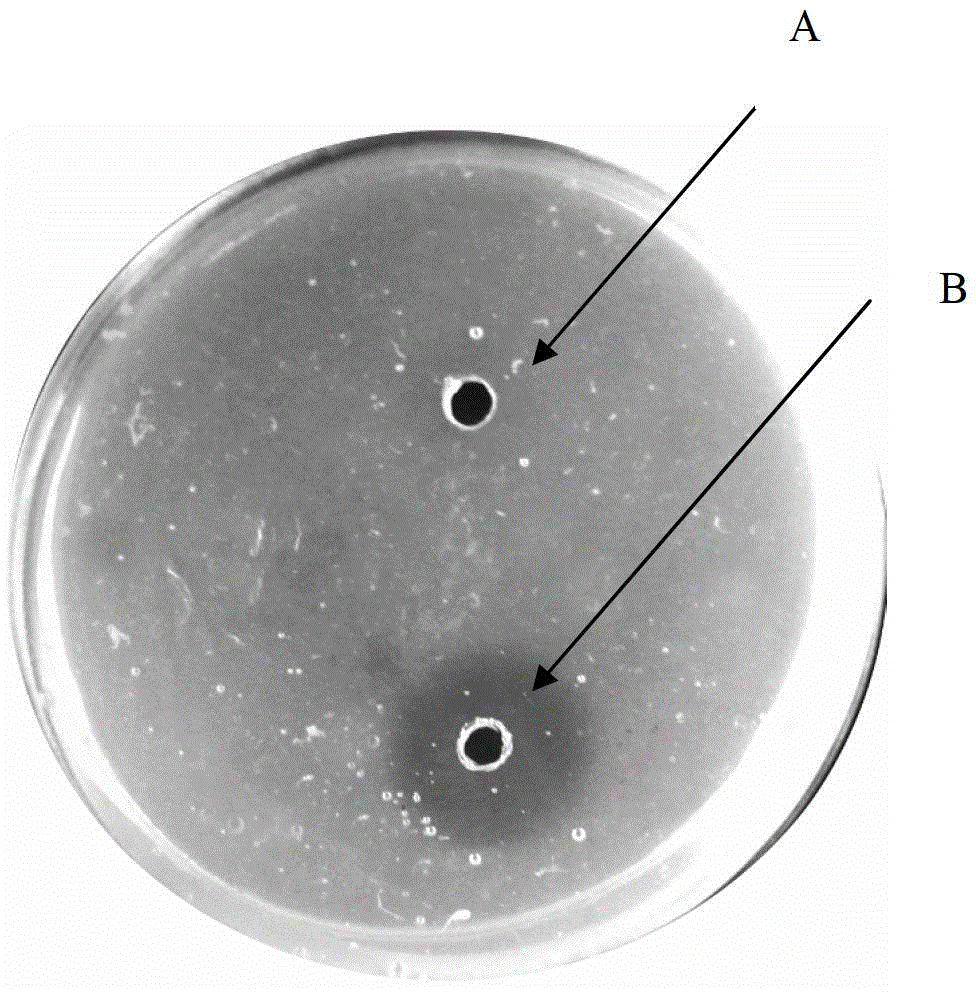
[0052] Slightly improved with reference to the Astrup fibrin plate method, its specific operation process: Weigh 1g of agarose and place it in a 100mL Erlenmeyer flask, add 45mL of 0.02mol / L Tris-HCl buffer solution (pH7.4) to prepare 2% For the agar solution, boil the water bath until the agarose is completely melted, then put it in a constant temperature water bath at 55°C. Take 1mL of thrombin (100U / mL) and dilute it to 2.5mL, weigh 50mg of fibrin and dissolve it in 2.5mL of 0.02mol / L Tris-HCl buffer (pH7.4). Add the thrombin and fibrin solution to the agarose in a water bath at 55°C, stir evenly, pour it into a sterilized 9cm petri dish, and cool it at room temperature to form a gel, which is a standard plate. A hole with a diameter of 0.5 cm was punched on the plate with a puncher.
[0053] Plasmin activity assay :
[0054] Make a standard plate, punch 7 holes with a...
Embodiment 3
[0055] "Example 3" Determination of the N-terminal Amino Acid Sequence of the PFE N-terminal Amino Acid Sequence
[0056] After SDS-PAGE electrophoresis, use 10×CAPS transfer buffer (100mmol / L) for protein transfer: Take 22.13g of CAPS, add 900mL of deionized water, adjust the pH to 11.0 with 2mol / L NaOH, and then constant volume to 1L and stored at 4°C. When transferring, take 200mL of 10×CAPS transfer buffer, 200mL of methanol, and 1600mL of deionized water. Transfer process: put the gel and PVDF membrane in a transfer tank containing pre-cooled transfer buffer, and transfer for 1.5h under the condition of a voltage of 200V. Take out the PVDF membrane and rinse it slightly with deionized water, soak it in methanol for a few seconds, then stain it with 0.1% Coomassie Brilliant Blue R-250, decolorize it with 50% methanol, and wash it thoroughly with deionized water. The purified enzyme components were subjected to SDS-PAGE electrophoresis, showing a single protein band aroun...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com