Catalyst for photo-catalytically decomposing water to produce hydrogen and preparation method of catalyst
A water splitting and photocatalytic technology, applied in the field of photocatalysis, can solve problems such as easy agglomeration, decrease in specific surface area, and decrease in photocatalytic performance, and achieve the effects of increasing the degree of dispersion, increasing the rate of hydrogen production, and simple preparation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
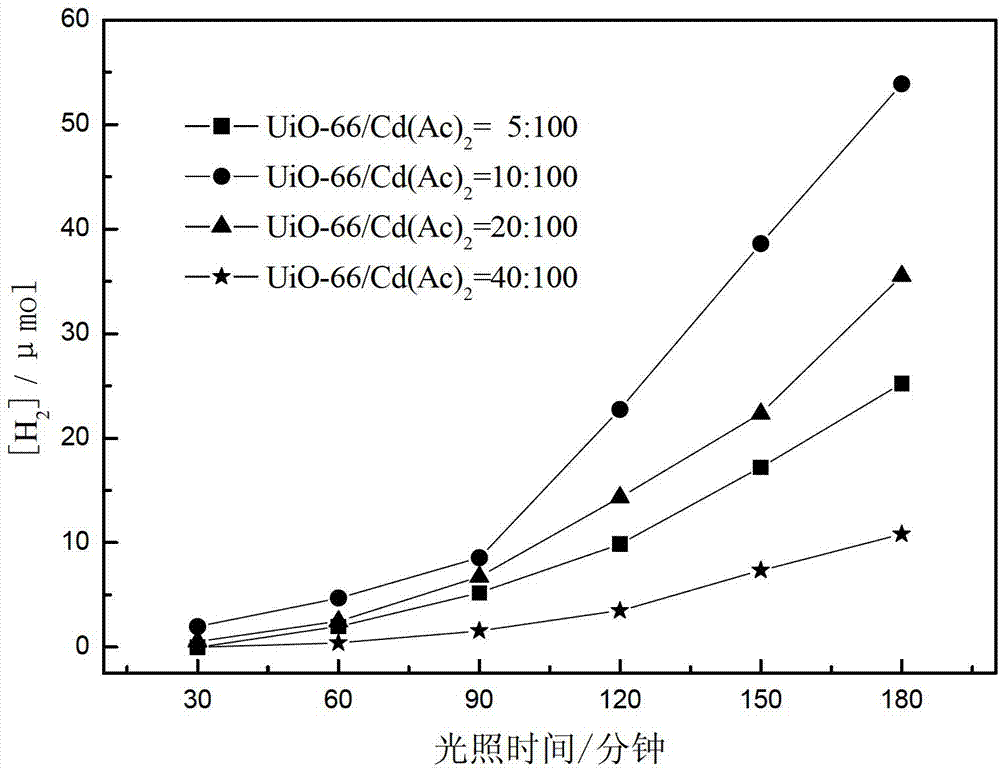
[0053] This example provides four photocatalytic water splitting catalysts CdS / UiO-66 for hydrogen production, which are prepared by the following steps:
[0054] Weigh 0.0032g, 0.0064g, 0.0128g, 0.0256g of activated UiO-66 respectively, add them to 24mL of dimethyl sulfoxide solvent, stir in a water bath at 25°C for 30 minutes, then weigh 0.064g Four parts of cadmium acetate were added to the above dimethyl sulfoxide solutions with different UiO-66 contents, and the stirring was continued for 3 hours, then transferred to a 30mL autoclave containing a polytetrafluoroethylene liner, and heated at 180 ℃ oven at constant temperature for 12 hours, naturally cooled to room temperature, washed several times with ethanol, and then put the product into a high-pressure reactor filled with 24mL ethanol at 80°C for 12 hours, and finally centrifuged the product at 100°C After drying for 12 hours, the mass ratios of precursor UiO-66 to cadmium acetate were 5:100, 10:100, 20:100 and 40:100,...
Embodiment 2
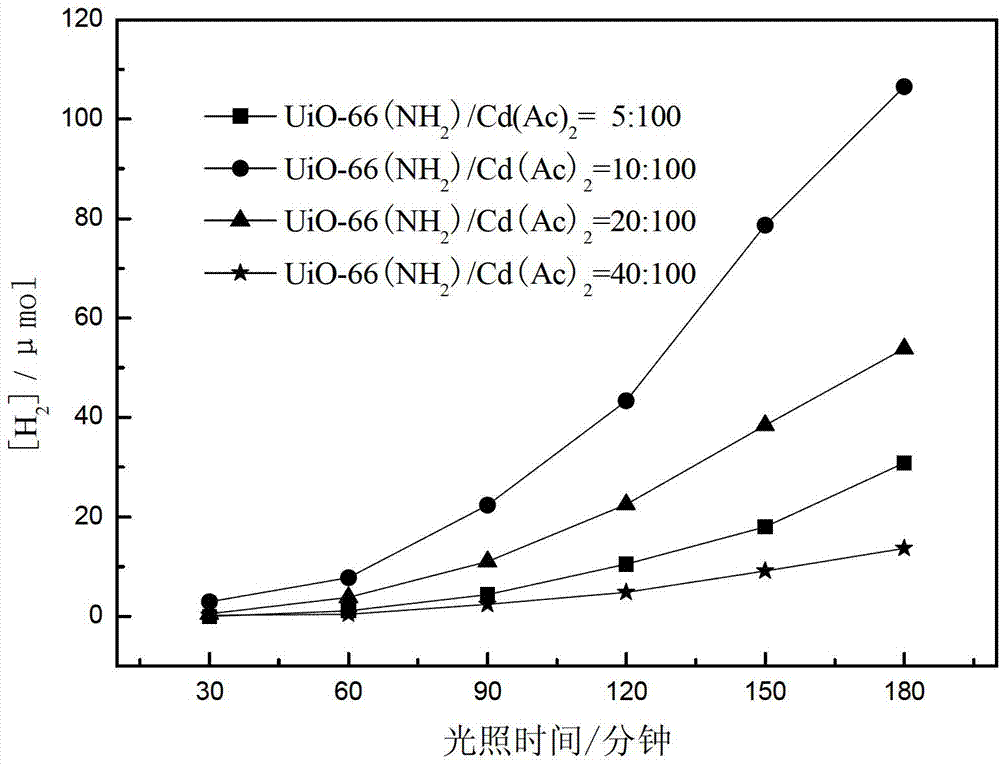
[0057] This example provides four kinds of catalysts CdS / UiO-66(NH 2 ), which was prepared by the following steps:
[0058] UiO-66 in embodiment 1 is changed into UiO-66 (NH 2 ), other conditions are the same as in Example 1, and the precursor UiO-66 (NH 2 ) to cadmium acetate with mass ratios of 5:100, 10:100, 20:100 and 40:100, respectively, the photocatalytic water splitting catalyst CdS / UiO-66(NH 2 ).
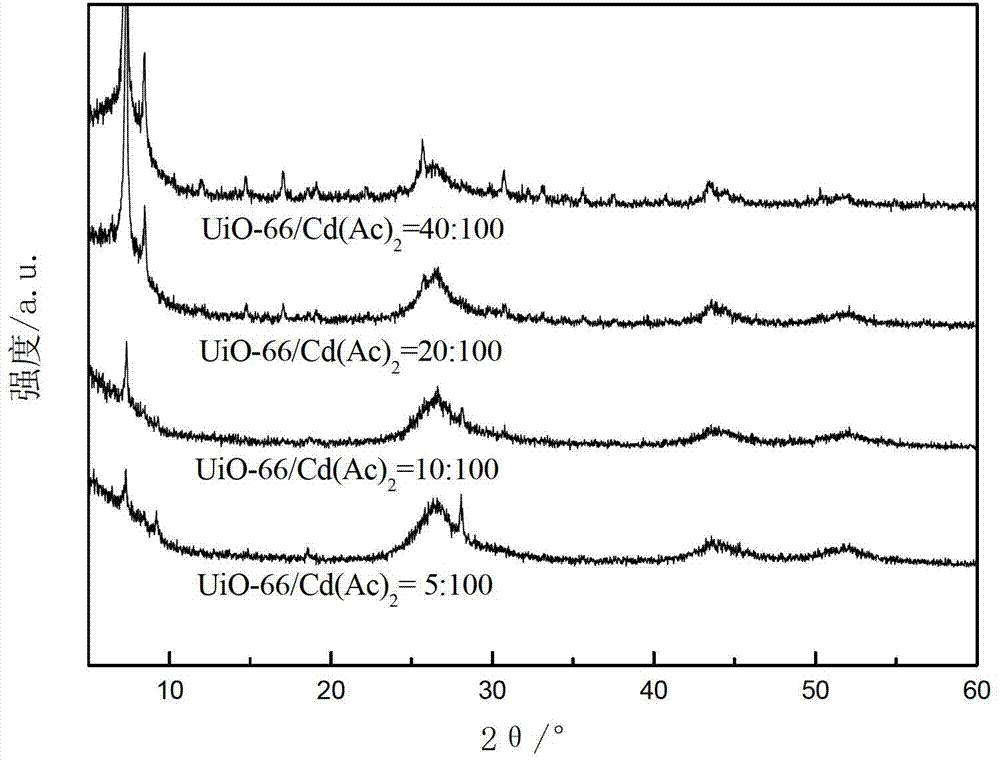
[0059] The XRD patterns of these four photocatalytic water splitting catalysts for hydrogen production are as follows: Figure 4 shown by Figure 4 It can be seen that UiO-66(NH 2 ) diffraction peaks, indicating that the above-mentioned photocatalytic water splitting catalyst for hydrogen production contains UiO-66(NH 2 ) phase, and the diffraction peak of CdS appeared at a high angle, indicating that CdS exists in the above-mentioned photocatalytic water splitting catalyst for hydrogen production.
Embodiment 3
[0060] Example 3 Photocatalytic water splitting activity evaluation
[0061] Using the Labsolar-II instrument of Profil Company to carry out the evaluation experiment of photocatalytic decomposition of water and Mars on the catalyst, the evaluation experiment is carried out in the following way:
[0062] Weigh 0.01g of catalyst and dissolve it in a beaker containing 100mL of distilled water, then weigh 4.50g of sodium sulfide and 2.50g of sodium sulfite as sacrificial reagents and dissolve in it, then place the beaker on a magnetic stirrer to stir for dispersion, and finally put it into an ultrasonic instrument , sonicate for 20 minutes under the condition of 90Hz;
[0063] After the ultrasonic treatment is completed, the reactants in the beaker are transferred to the quartz reactor of the photocatalytic device, and the entire photocatalytic device is vacuumed before the reaction starts, which needs to reach minus one atmospheric pressure;
[0064] The xenon light source is u...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com