A method for predicting the risk of a subject for contracting diabetes mellitus and/or metabolic syndrome or for diagnosing metabolic syndrome in a subject
A technology for metabolic syndrome and diabetes, which is applied in disease diagnosis, metabolic disease, biological testing, etc., and can solve problems such as undescribed correlations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0086] Antibody preparation
[0087] Peptides / Conjugates for Immunization
[0088] Peptides for immunization were synthesized (JPT Technologies, Berlin, Germany) with an additional N-terminal cysteine residue to couple the peptide to bovine serum albumin (BSA). The peptide was covalently coupled to BSA by using Sulfo-SMCC (Perbio-science, Bonn, Germany). Coupling steps were performed according to Perbio's manual.
[0089] Labeled Antibody (LA) Peptides (P-NT 1-19):
[0090] H-CSDSEEEMKALEADFLTNMH-NH2
[0091] Solid Phase Antibody (SPA) Peptide (P-NT 44-62):
[0092] H-CNLNSPAEETGEVHEEELVA-NH2
[0093] Generate antibodies as follows:
[0094] BALB / c mice were immunized with 100 μg of peptide-BSA conjugate on days 0 and 14 (emulsified in 100 μl complete Freund’s adjuvant) and 50 μg on days 21 and 28 (in 100 μl in incomplete Freund's adjuvant). Animals received 50 μg of conjugate dissolved in 100 μl of saline by one intraperitoneal injection and one intravenous injectio...
Embodiment 2
[0101] Immunoassay for quantification of human proneurotensin
[0102] The technique used is based on acridinium ester labeled sandwich-coated tube luminescence immunoassay.
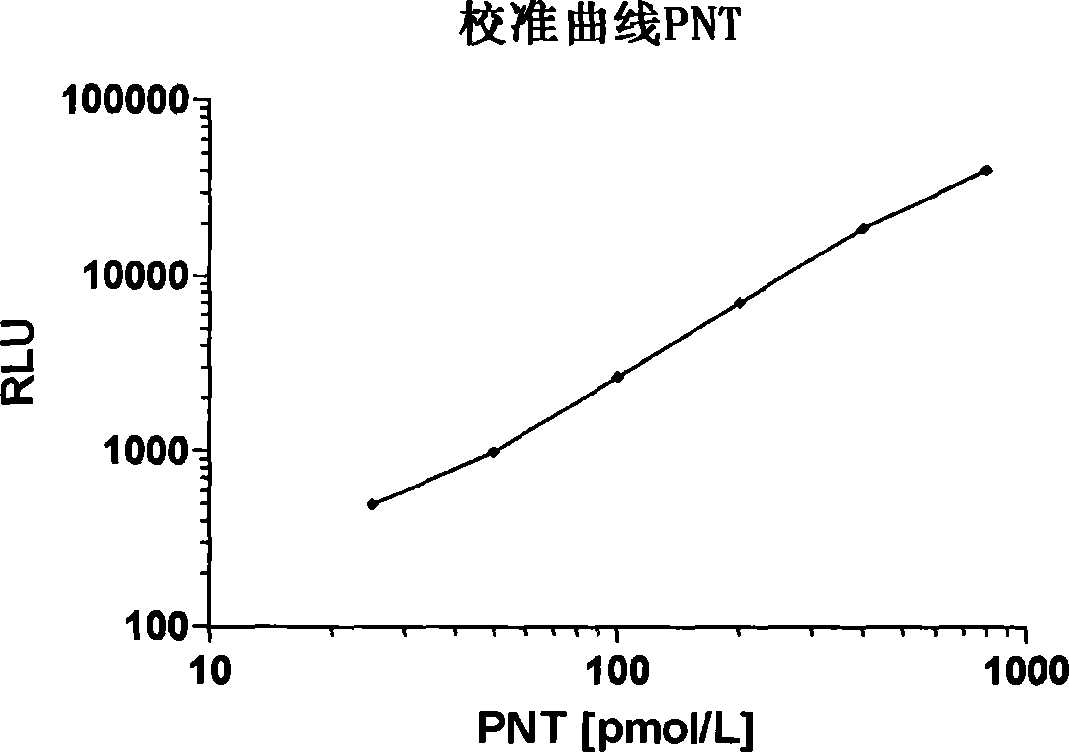
[0103] Labeled complex (tracer): 100 μg (100 μl) LA (1 mg / ml dissolved in PBS, pH 7.4) was mixed with 10 μl acridine NHS ester (1 mg / ml dissolved in acetonitrile, InVent GmbH, Germany) (EP 0353971 ) and incubate at room temperature for 20 minutes. Labeled LA was purified by gel filtration HPLC on Bio-Sil SEC 400-5 (Bio-Rad Laboratories, Inc., USA). Purified LA was diluted in (300 mmol / l potassium phosphate, 100 mmol / l NaCl, 10 mmol / l Na-EDTA, 5 g / l bovine serum albumin, pH 7.0). The final concentration was about 800.000 relative light units (RLU) of labeled complex (about 20 ng of labeled antibody) per 200 μl. Acridinium ester chemiluminescence was measured with AutoLumat LB 953 (Berthold Technologies GmbH & Co. KG).
[0104] Solid phase: Polystyrene tubes (Greiner Bio-One International AG, Austria) ...
Embodiment 3
[0113] group study
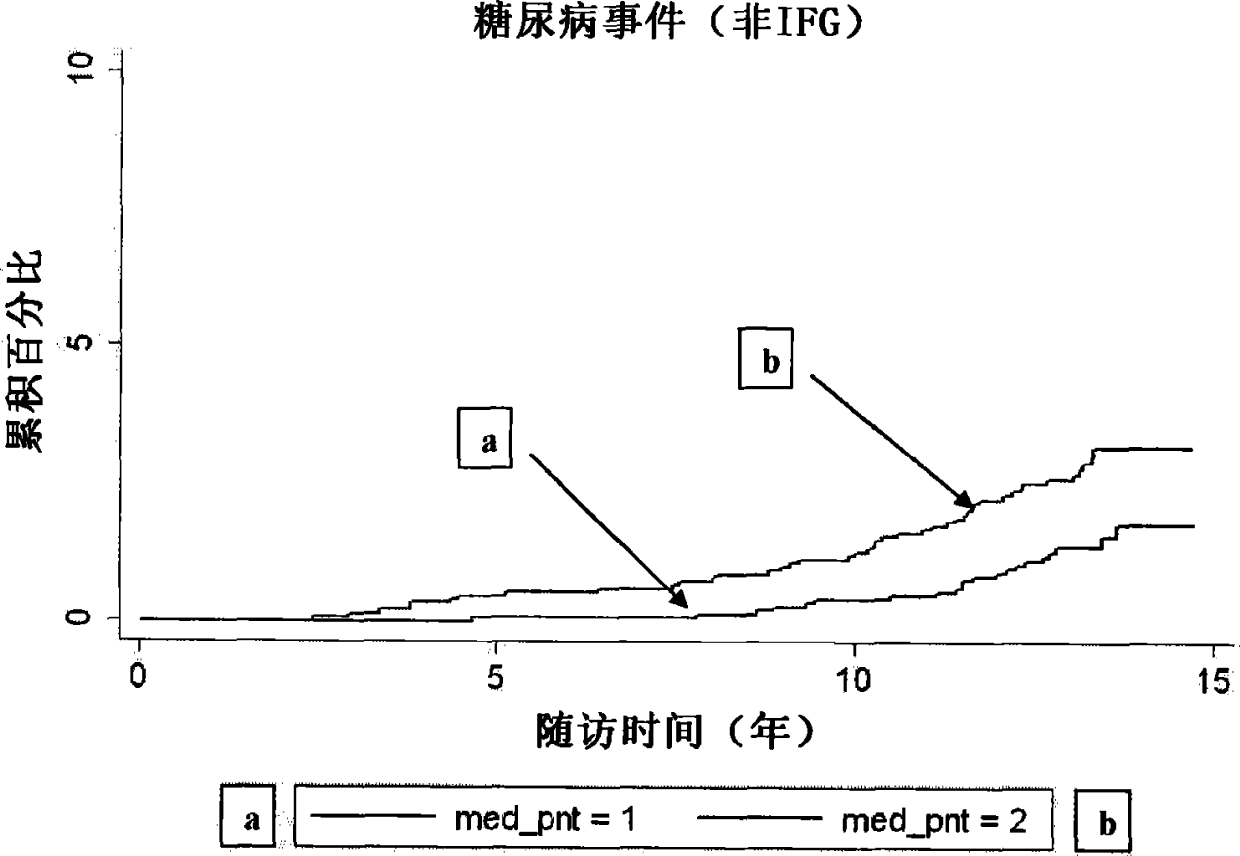
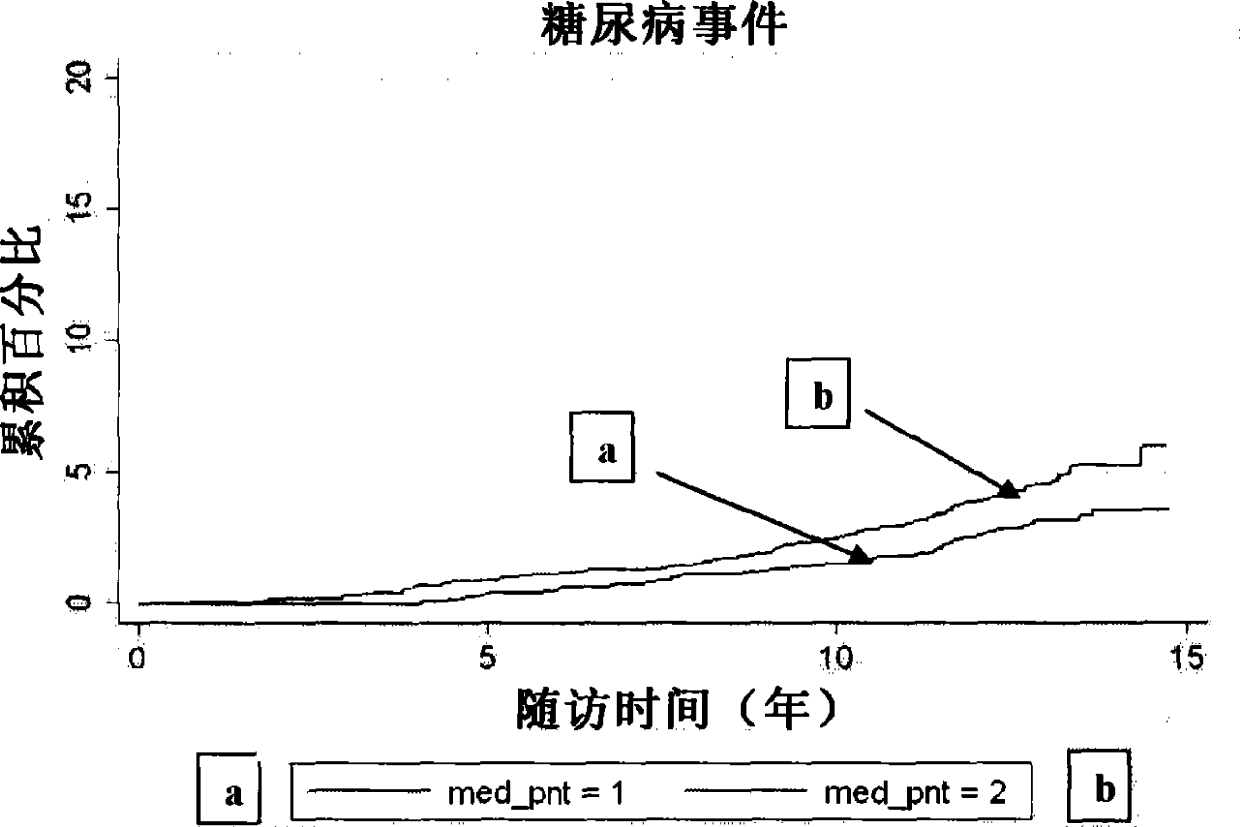
[0114] We measured P-NT in fasting plasma from 4362 participants (mean age 58±6 years, 59% female) who underwent the baseline examination of the Malmö Diet and Cancer Study in 1991-1994. crowd. We compared baseline P-NT (to log-transformed P- Hazard ratio per one standard deviation increase in NT) was associated with time to first event for each study endpoint, with a median follow-up of more than 12 years. Endpoints were retrieved from the Swedish National Hospital Discharge Register, the Swedish Myocardial Infarction Register, the Malmö Stroke Register, and the Swedish Cancer Register. Endpoints retrieved through these registries were validated and found to be accurate.
[0115] Table 1
[0116] Clinical characteristics of the total study population
[0117] descriptive statistics
[0118]
[0119] Table 2
[0120] gender
[0121]
[0122] table 3
[0123] Questionnaire + diary records: Antihypertensive treatment at baseline obtained f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com