A kind of preparation method of proton exchange membrane fuel cell catalyst
A proton exchange membrane, fuel cell technology, applied in chemical instruments and methods, physical/chemical process catalysts, battery electrodes, etc. Anti-methanol and methanol oxidation intermediates poisoning, solve the problem of catalyst resources, improve the effect of stability and life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
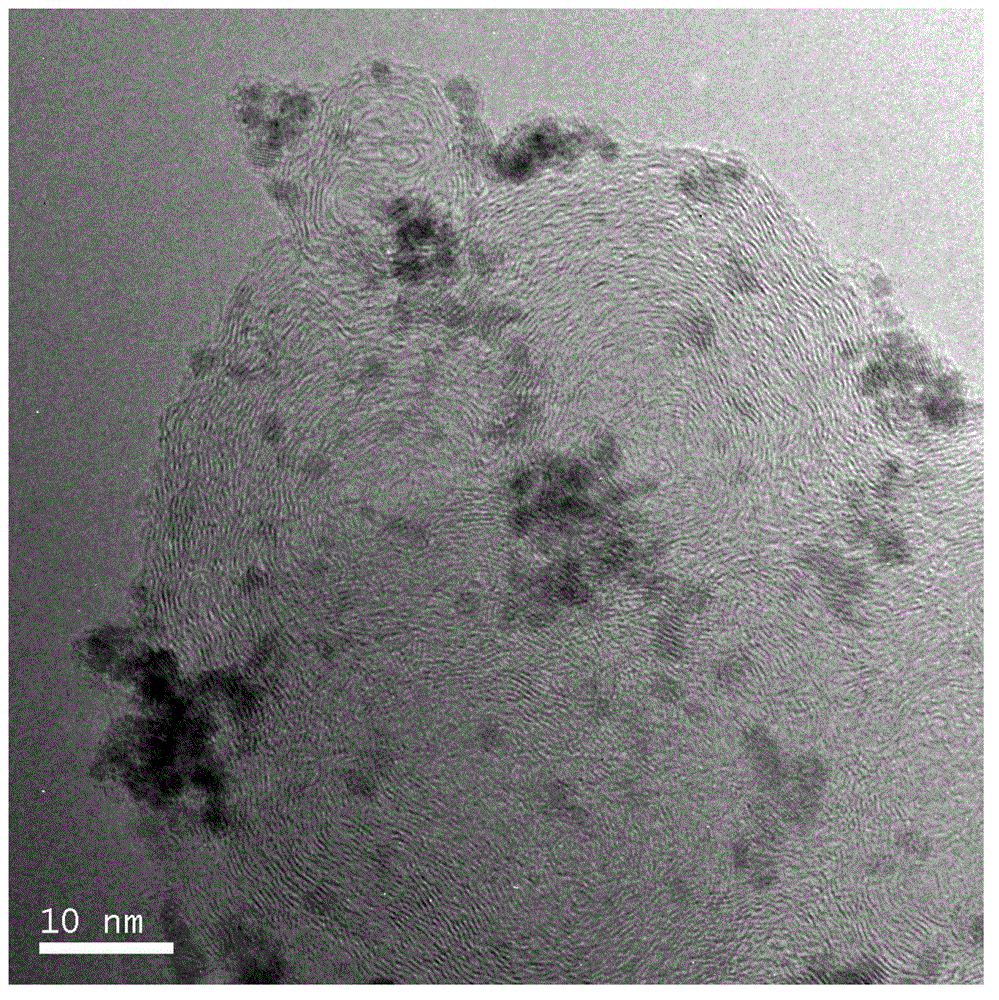
[0026] In the first step, by reducing the CoCl 2 ·6H 2 O to prepare Co particles. At 0.1 mol / L CoCl 2Add 10ml of polyvinylpyrrolidone (PVP) ethanol solution to the ethanol solution as a stabilizer, slowly add 0.05mol / L NaBH under stirring 4 Add the ethanol solution to the above mixed solution, and pass nitrogen gas to prevent the particles from being oxidized. Add NaBH 4 After reacting for 1 h with the ethanol solution, the solid and liquid were separated, and the product was washed with water to obtain Co nanoparticles.
[0027] In the second step, the reduction of RuCl with ethylene glycol 3 CoRu core-shell nanoparticles were prepared with Co particle suspension solution. The Co particles prepared in the previous step were dispersed in 20ml of ethylene glycol, and a certain amount of PVP was added as a stabilizer under stirring. After stirring for 30min, 0.05 mol / L RuCl was added dropwise 3 ethylene glycol solution. After mixing evenly, adjust the pH value to 11 with...
Embodiment 2
[0033] In the first step, by reducing the CoCl 2 ·6H 2 O to prepare Co particles. At 0.1 mol / L CoCl 2 Add 10ml of polyvinylpyrrolidone (PVP) ethanol solution to the ethanol solution as a stabilizer, slowly add 0.05mol / L NaBH under stirring 4 Add the ethanol solution to the above mixed solution, and pass nitrogen gas to prevent the particles from being oxidized. Add NaBH 4 After reacting for 1 h with the ethanol solution, the solid and liquid were separated, and the product was washed with water to obtain Co nanoparticles.
[0034] In the second step, the reduction of RuCl with ethylene glycol 3 CoRu core-shell nanoparticles were prepared with Co particle suspension solution. The Co particles prepared in the previous step were dispersed in 20ml of ethylene glycol, and a certain amount of PVP was added as a stabilizer under stirring. After stirring for 30min, 0.05 mol / L RuCl was added dropwise 3 ethylene glycol solution. After mixing evenly, adjust the pH value to 12 wit...
Embodiment 3
[0040] In the first step, by reducing the CoCl 2 ·6H 2 O to prepare Co particles. At 0.1 mol / L CoCl 2 Add 10ml of polyvinylpyrrolidone (PVP) ethanol solution to the ethanol solution as a stabilizer, slowly add 0.05mol / L NaBH under stirring 4 Add the ethanol solution to the above mixed solution, and pass nitrogen gas to prevent the particles from being oxidized. Add NaBH 4 After reacting for 1 h with the ethanol solution, the solid and liquid were separated, and the product was washed with water to obtain Co nanoparticles.
[0041] In the second step, the reduction of RuCl with ethylene glycol 3 CoRu core-shell nanoparticles were prepared with Co particle suspension solution. The Co particles prepared in the previous step were dispersed in 20ml of ethylene glycol, and a certain amount of PVP was added as a stabilizer under stirring. After stirring for 30min, 0.05 mol / L RuCl was added dropwise 3 ethylene glycol solution. After mixing evenly, adjust the pH value to 12 wit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com