Method and device for producing 2, 2, 6, 6-Tetramethyl-4-piperidinol through continuous catalytic hydrogenation
A technology for catalytic hydrogenation of tetramethylpiperidinol, which is applied in organic chemistry and other fields, can solve problems such as rarely used, complicated treatment, and high energy consumption in production, so as to increase the effect of vapor-liquid mass transfer, promote the progress of reaction, increase The effect of vapor-liquid ratio
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
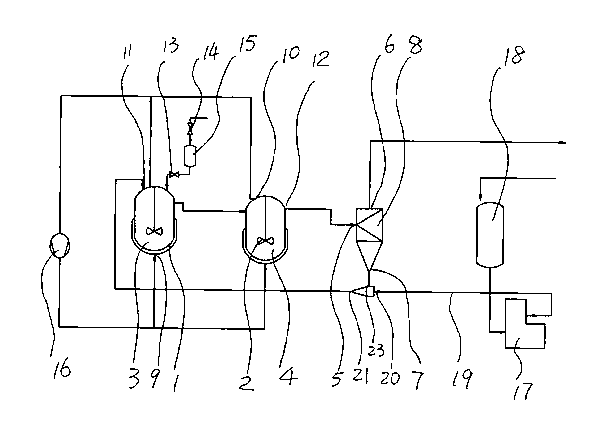
[0037] The structure and operating principle of the continuous catalytic hydrogenation production tetramethylpiperidinol device provided by the present invention will be further described in detail below in conjunction with the accompanying drawings.
[0038] Such as figure 1 The above is a structural schematic diagram of a two-stage continuous catalytic hydrogenation device for producing tetramethylpiperidinol consisting of two reactors connected in series. The structure constituting the device includes a primary reactor 3 and a secondary reactor 4 respectively provided with a heating and cooling mechanism 1 and a stirring mechanism 2, a tangential material inlet 5 is provided in the middle, a finished product outlet 6 is provided in the upper part, and a finished product outlet 6 is provided in the lower part. There is a rotary sedimentation separator 8 composed of a sediment outlet 7, wherein the bottom of the two-stage reactor is respectively provided with a hydrogen inlet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com