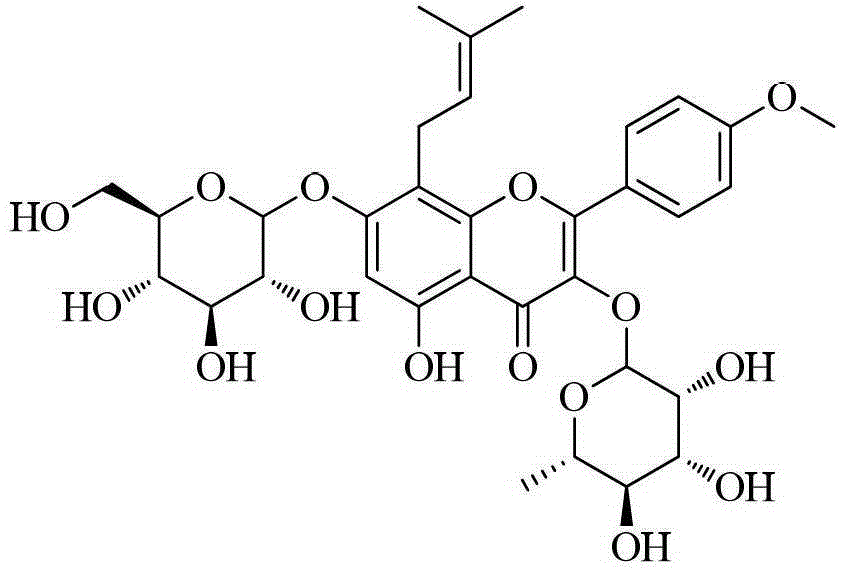
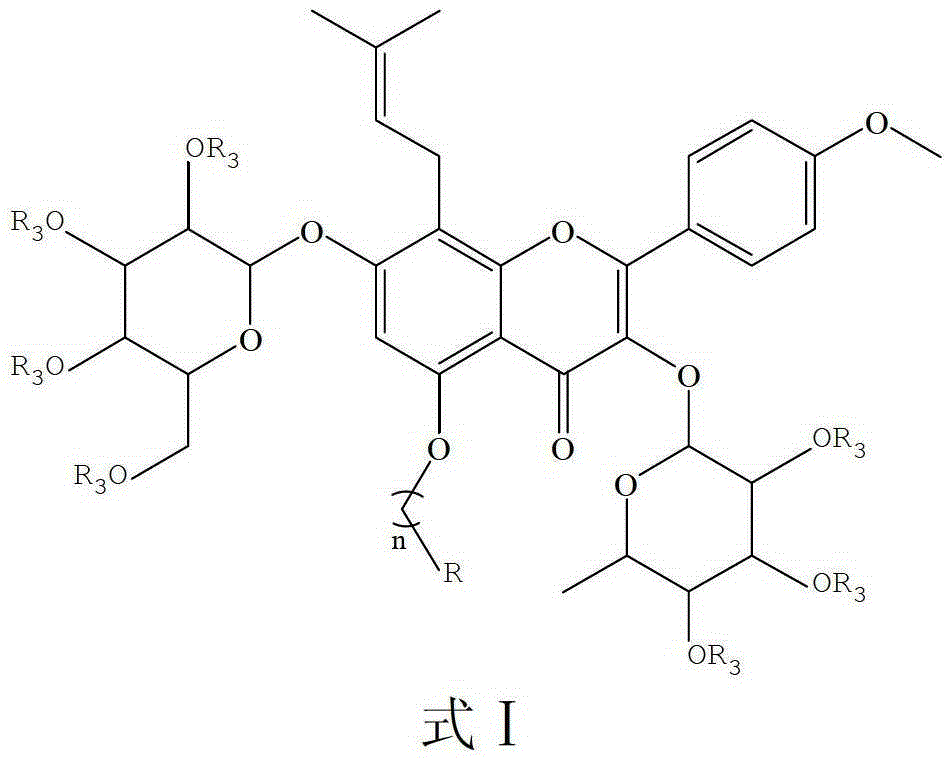
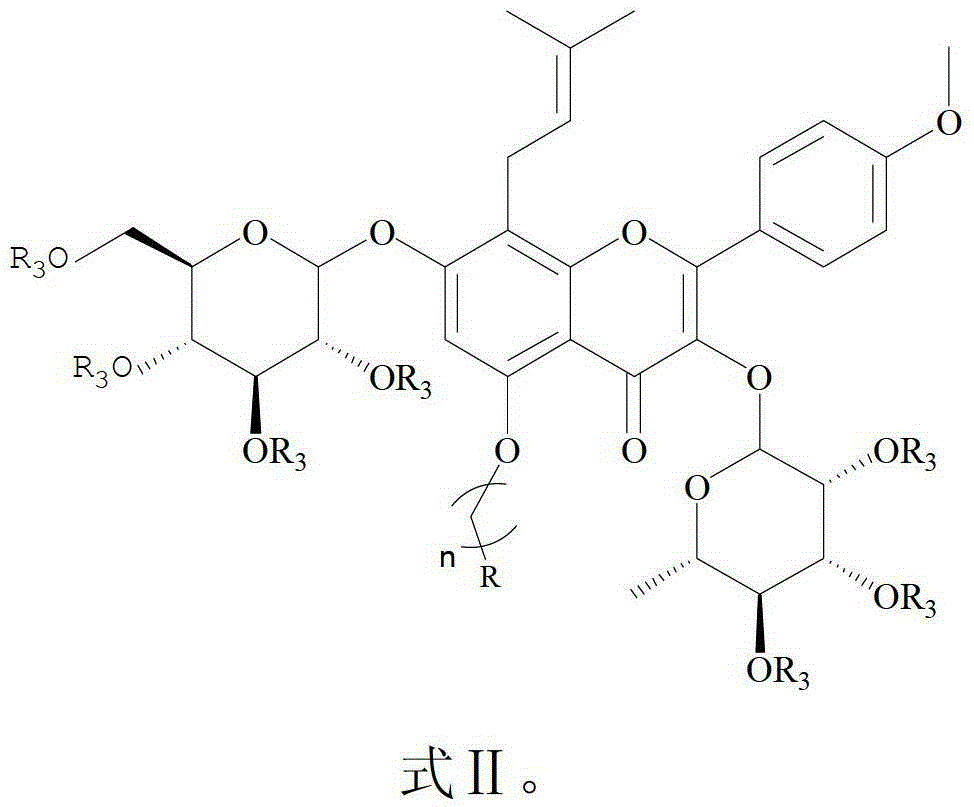
Icariin Derivatives
A technology of icariin and its derivatives, which is applied in the preparation of sugar derivatives, sugar derivatives, sugar derivatives, etc., to achieve the effect of excellent drug effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1 Preparation of Icariin Derivatives (Compounds 7, 8) of the present invention
[0036] Dissolve 5 g (7.4 mmol, 1 equiv) of ICA in 30 mL of DMF, add 5.7 g (51.8 mmol, 7 equiv) of triethylamine under ice-bath conditions, stir for 10 min, add 5.7 g (51.8 mmol, 7 equiv) of acetic anhydride dropwise, and Stir for 6 h, quench with ice water, filter, wash the solid with water, dissolve in ethyl acetate, dry over anhydrous MgSO4, concentrate in vacuo, and column chromatography to obtain an alcoholic hydroxyl-protected icariin derivative (1 g, 15%). Dissolve 0.5 g (0.52 mmol, 1 equiv) of the protected product in 2 mL of anhydrous acetone, add appropriate amount of anhydrous K2CO3, catalytic amount of KI, stir for 10 min, add 0.55 mmol (1.1 equiv) of 1,3-dibromopropane or Ethyl bromoacetate, stirred at room temperature for 1-3h, and the reaction was monitored by TLC. After the reaction was completed, the solid was removed by filtration, and the filtrate was concentrated ...
Embodiment 2
[0039] Example 2 Synthesis of Nitrogenous Derivatives of Icariin (Compounds 9 and 10)
[0040] Take 0.19g (0.17mmol, 1equiv) of compound 7 and dissolve it in anhydrous acetone, add an appropriate amount of K 2 CO 3 and catalytic amount KI, stirred at room temperature for 10 min, added piperidine or piperazine, stirred at room temperature for 30 min, filtered to remove solids, and the filtrate was concentrated in vacuo. The crude product was purified by column chromatography to obtain products 9 and 10.
[0041]Compound 9: yellow powder, yield 90%.Mp.120~121℃; 1HNMR (400MHz, CDCl3) δ7.82(d, 2H, J=8.80Hz), 7.06(d, 2H, J=8.80Hz), 6.92(s,1H),5.67(t,1H,J=2.08Hz),5.52(d,1H,J=7.04Hz),5.45(s,1H),5.32~5.40(m,2H),5.23~5.03 (m,2H),5.06(t,1H,J=6.56Hz),4.96(t,1H,J=10.08Hz),4.64(bs,1H),4.39~4.31(m,4H),3.90(s, 3H), 3.71~3.77(bs, 4H), 3.38~3.48(m, 2H), 2.50(d, 2H, J=5.12Hz), 2.13(s, 3H), 2.03(s, 3H), 2.00(d ,6H,J=3.84Hz),1.68(s,3H),1.66(s,3H),1.63(s,15H),1.22~1.26(m,3H),0.81(d,3H,J=6.24Hz...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com