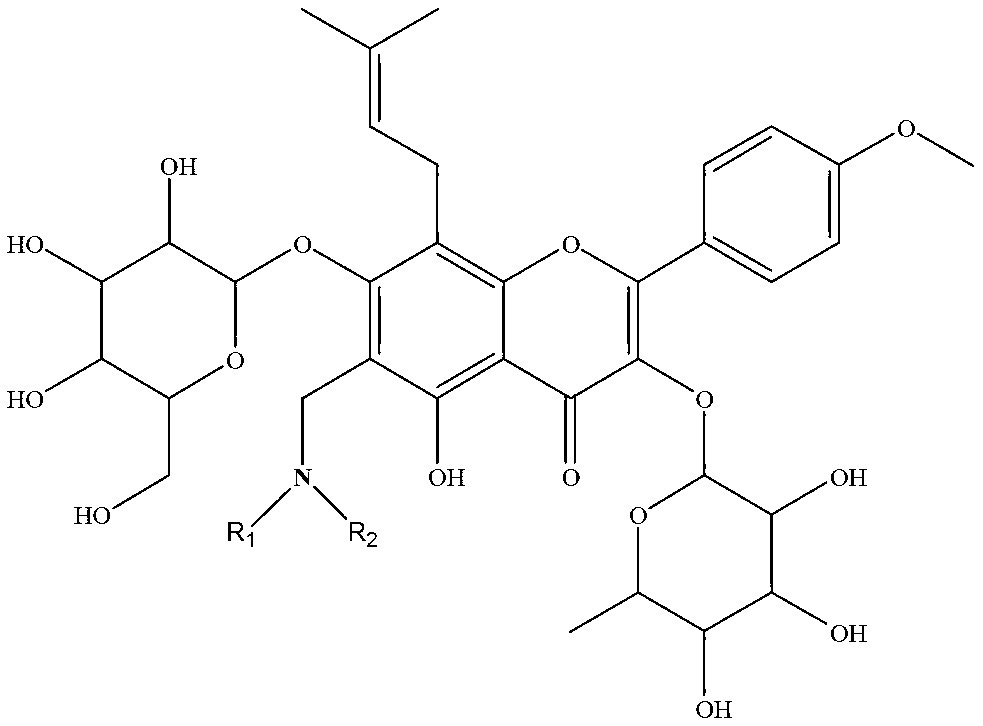
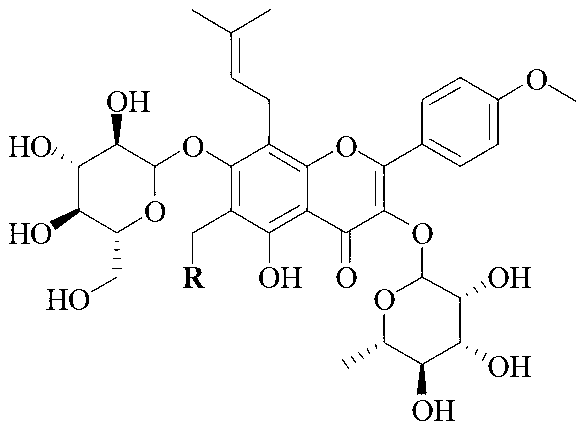
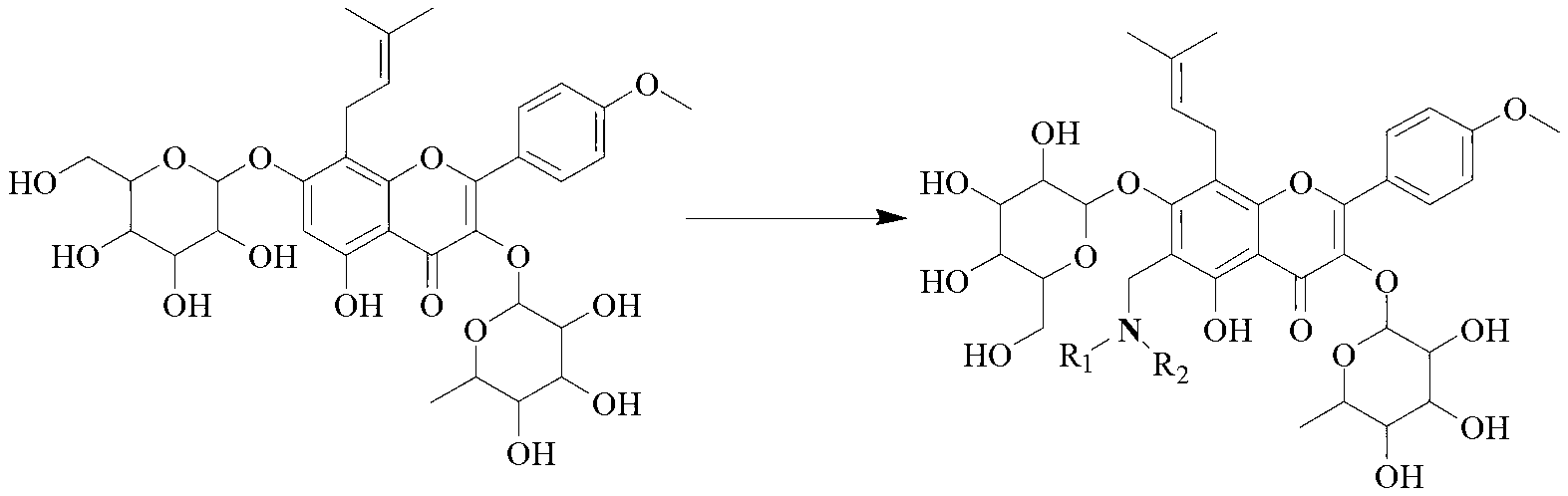
Icariin derivative, and preparation method and application thereof
A technology of icariin and derivatives is applied in the preparation of sugar derivatives, sugar derivatives, sugar derivatives, etc., to achieve the effect of excellent medicinal effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1 Preparation of icariin derivatives of the present invention
[0031] Take 0.5g (0.74mmol, 1equiv) ICA in a 50mL flask, add it in 10mL methanol and stir to form a suspension for later use, take another 50mL flask and add 37% formaldehyde solution (10equiv), 2mL methanol to dilute, add dropwise with stirring ( 0.74mmol, 1equiv) secondary ammonia (structural formula is as follows), stirred at room temperature for 10min, slowly added the methanol suspension of ICA into the flask, heated to reflux for 12h, thin-layer monitoring, after the reaction was completed, concentrated in vacuo, the crude product was dissolved. It was washed with ethyl acetate and saturated brine, then washed with water, dried over anhydrous MgSO4, concentrated in vacuo, and subjected to column chromatography to obtain the nitrogen-containing derivative of icariin corresponding to secondary ammonia.
[0032] The structural formula of above-mentioned secondary ammonia is where R 1 , R 2 is...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com