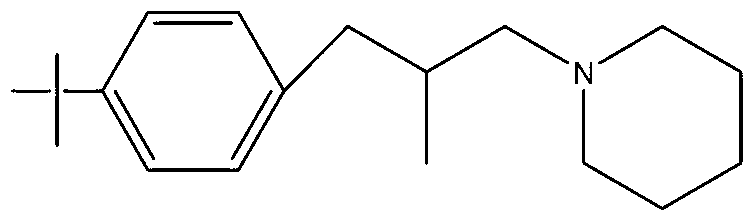
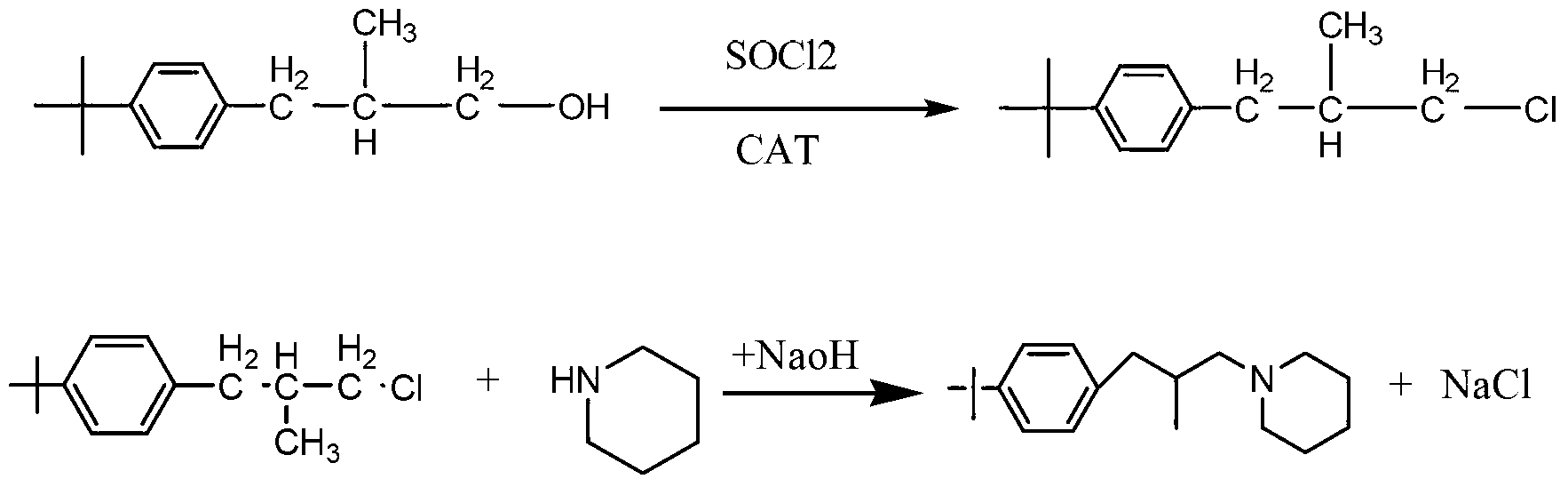
Synthesis method of Fenpropidin
A synthesis method and technology of fenprodrin are applied in the field of fenpropidin synthesis, and can solve problems such as low content of fenprodrin product, low yield, many side reactions and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Add 103g of p-tert-butyl-β-methylphenylpropanol to a 500ml round-bottomed flask, then add 0.5g of DMF, heat up to 40°C, add 62.5g of thionyl chloride dropwise, after the dropwise addition, keep warm at 90°C for 1 Hour. HCl, SO produced during the reaction process 2 The gas is absorbed in stages with water and 30% NaOH aqueous solution. After the heat preservation is finished, neutralize with liquid caustic soda to PH=8, and let stand to separate into layers. The upper layer is the reacted intermediate p-tert-butyl-β-methylphenylchloropropane. 113g of the intermediate was obtained by weighing, the analytical content (GC normalization method) was 98.2%, and the yield was 99.4%.
[0019] Put the p-tert-butyl-β-methylphenylchloropropane, the product of the first halogenation synthesis, into a 500ml four-neck flask, add 127.5g of piperidine, raise the temperature to 110°C, and keep it under reflux for 8 hours. After the sampling is qualified, use then Neutralize to PH = ...
Embodiment 2
[0021] Add 206g of p-tert-butyl-β-methylphenylpropanol to a 1000ml round bottom flask, then add 1g of DMF, raise the temperature to 40°C, add 125g of thionyl chloride dropwise, and keep the temperature at 90°C for 1 hour after the dropwise addition. HCl, SO produced during the reaction process 2 The gas is absorbed in stages with water and 30% NaOH aqueous solution. After the heat preservation is finished, neutralize with liquid caustic soda to PH=8, and let stand to separate into layers. The upper layer is the reacted intermediate p-tert-butyl-β-methylphenylchloropropane. Weighed 225g of the intermediate, the analytical content (GC normalization method) was 98%, and the yield was 99.2%.
[0022] Put p-tert-butyl-β-methylphenylchloropropane, the product of the first halogenation synthesis, into a 1000ml four-neck flask, add 255g of piperidine, heat up to 110°C, and keep at reflux for 8 hours. After the sampling is qualified, use 30% NaOH aqueous solution was neutralized to ...
Embodiment 3
[0024] Add 309g of p-tert-butyl-β-methylphenylpropanol to a 1000ml round bottom flask, then add 1.5g of DMF, raise the temperature to 40°C, add 187.5g of thionyl chloride dropwise, after the dropwise addition, keep warm at 90°C for 1 Hour. HCl, SO produced during the reaction process 2 The gas is absorbed in stages with water and 30% NaOH aqueous solution. After the heat preservation is finished, neutralize with liquid caustic soda to PH=8, and let stand to separate into layers. The upper layer is the reacted intermediate p-tert-butyl-β-methylphenylchloropropane. Weighed 336.5g of the intermediate, the analytical content (GC normalization method) was 98.2%, and the yield was 98.7%.
[0025] Put the p-tert-butyl-β-methylphenylchloropropane, the product of the first halogenation synthesis, into a 2000ml four-necked flask, add 382.5g of piperidine, raise the temperature to 110°C, and keep it under reflux for 8 hours. After the sampling is qualified, use then Neutralize to PH ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| refractive index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com