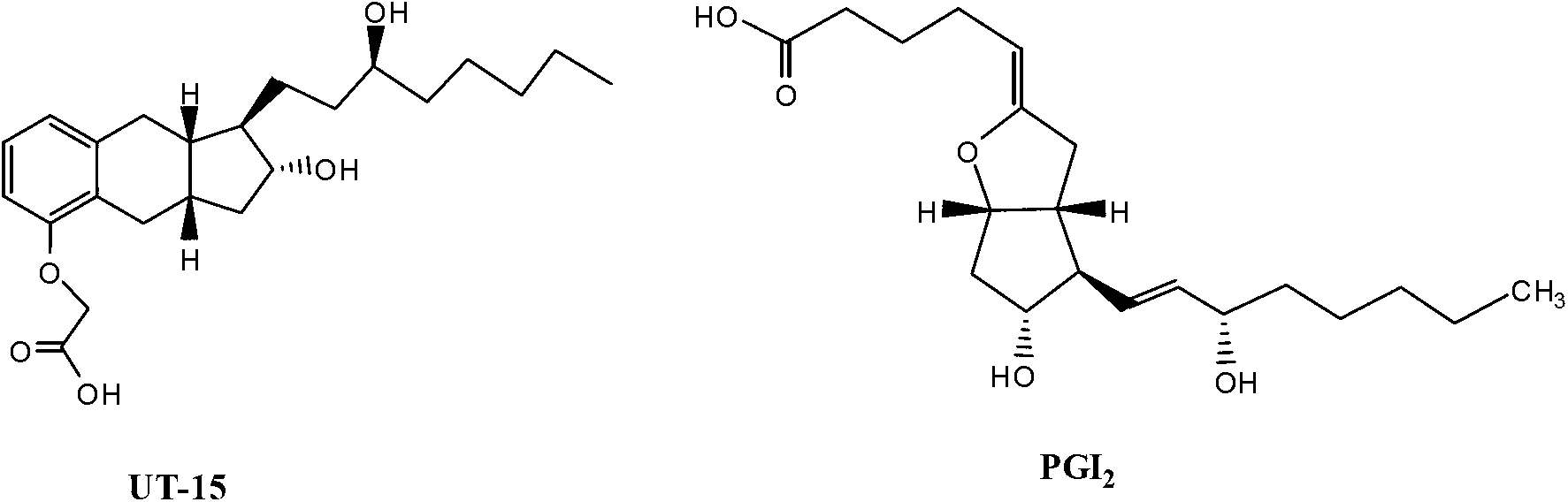
Treprostinil production
A technology of cyclic compounds and structural formulas, applied in the field of intermediate compounds, can solve the problems of lengthy chromatographic purification technology, expensive reagents, and no description of a feasible preparation method of treprostinil
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0043] The present application is directed to the preparation method of Treprostinil and the preparation method of the synthetic intermediate used for the synthesis of Treprostinil and the synthetic intermediate itself. The present application is also directed to a method of preparing treprostinil or a pharmaceutically acceptable salt thereof comprising the alkyne addition reaction described herein. Preferred treprostinil salts may include sodium and diethanolamine salts (see, eg, US Patent No. 7,417,070).
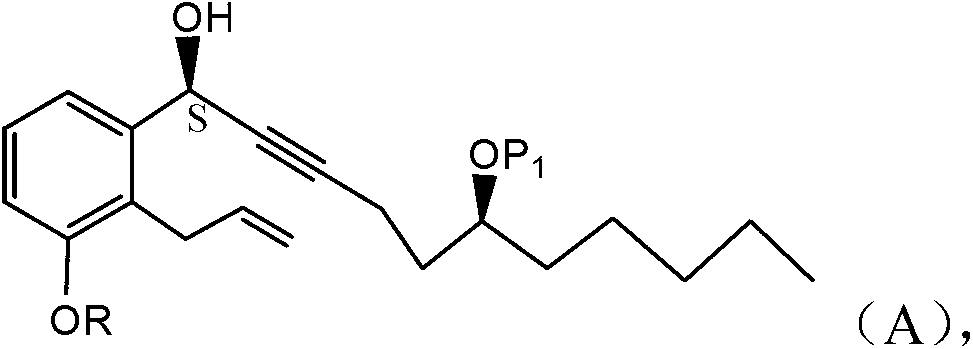
[0044] In some embodiments, the present application is directed to a method for preparing treprostinil synthetic intermediate (A) through stereoselective alkyne addition reaction.
Embodiment approach
[0045] One embodiment is directed to a novel process (reaction 1) for the preparation of compounds of formula (A) comprising the step of reacting an aldehyde of formula (I) with an alkyne of formula (a):
[0046]
[0047] in:
[0048] P 1 is an alcohol protecting group;
[0049] R is -(CH 2 ) n X;
[0050] X is H, phenyl, -CN, -OR 1 or COOR 1 ;
[0051] R 1 is alkyl, THP, TBDMS, or substituted or unsubstituted benzyl; and
[0052] n is 1, 2 or 3.
[0053]As used herein, an "alcohol protecting group" is a functional group that protects an alcohol group from reactions occurring with other parts of the molecule. Suitable alcohol protecting groups are known to those skilled in the art and include those known from the following document and the entire teaching of which document is incorporated herein by reference: T.W. Greene, Protecting Groups in Organic Synthesis, John Wiley & Sons, Inc. 1981. Exemplary alcohol protecting groups include, but are not limited to, ace...
Embodiment 1
[0125] Example 1. Preparation of Chiral Benzyl Alcohol (A-1)
[0126]
[0127] Zinc triflate (2.16 g, 0.0059 mol) and (+)-N-methylephedrine (0.814 g, 0.0045 mol) in toluene (10 mL) were charged to a 50 mL bismuth equipped with a mechanical stirrer. necked round bottom flask. Triethylamine (0.459 g, 0.0045 mol) was added to the mixture and the gel-like mixture was stirred at ambient temperature for 30-60 minutes. The mixture was then treated with a solution of the alkyne (1.08 g, 0.0045 mol) in toluene (1 mL), stirred at ambient temperature for 15 minutes, followed by a solution of acetaldehyde (0.250 g, 0.0014 mol). The progress of the reaction was monitored by TLC (completion of the reaction was monitored by TLC using a thin-layer silica gel plate; eluent: 20% ethyl acetate in hexane). After stirring the mixture for 3 hours, TLC indicated the reaction was complete. At this stage, the reaction mixture was quenched by the slow addition of saturated ammonium chloride (10 m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com