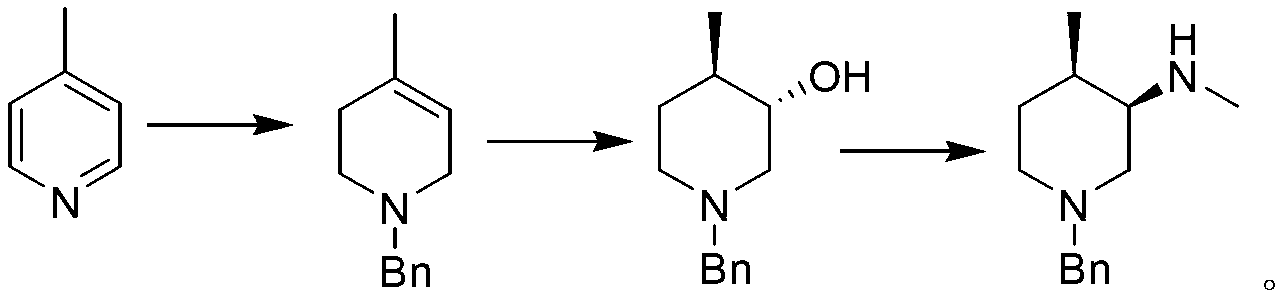
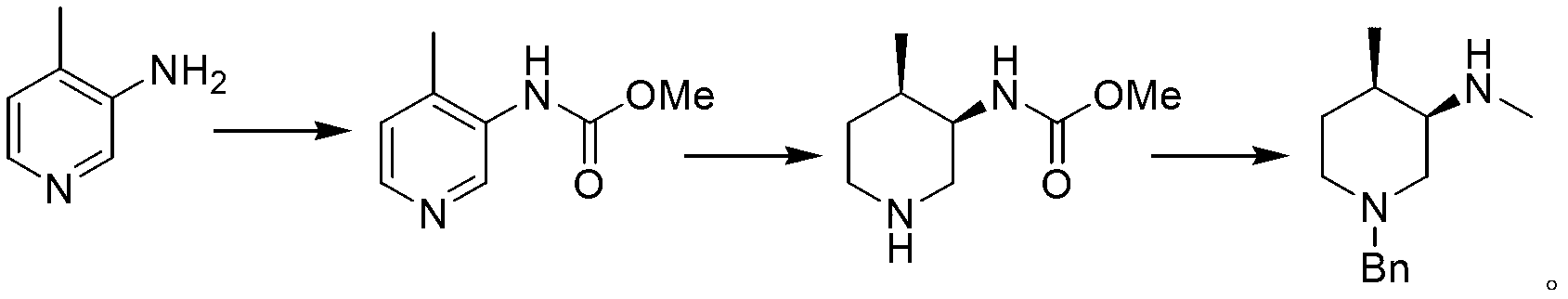
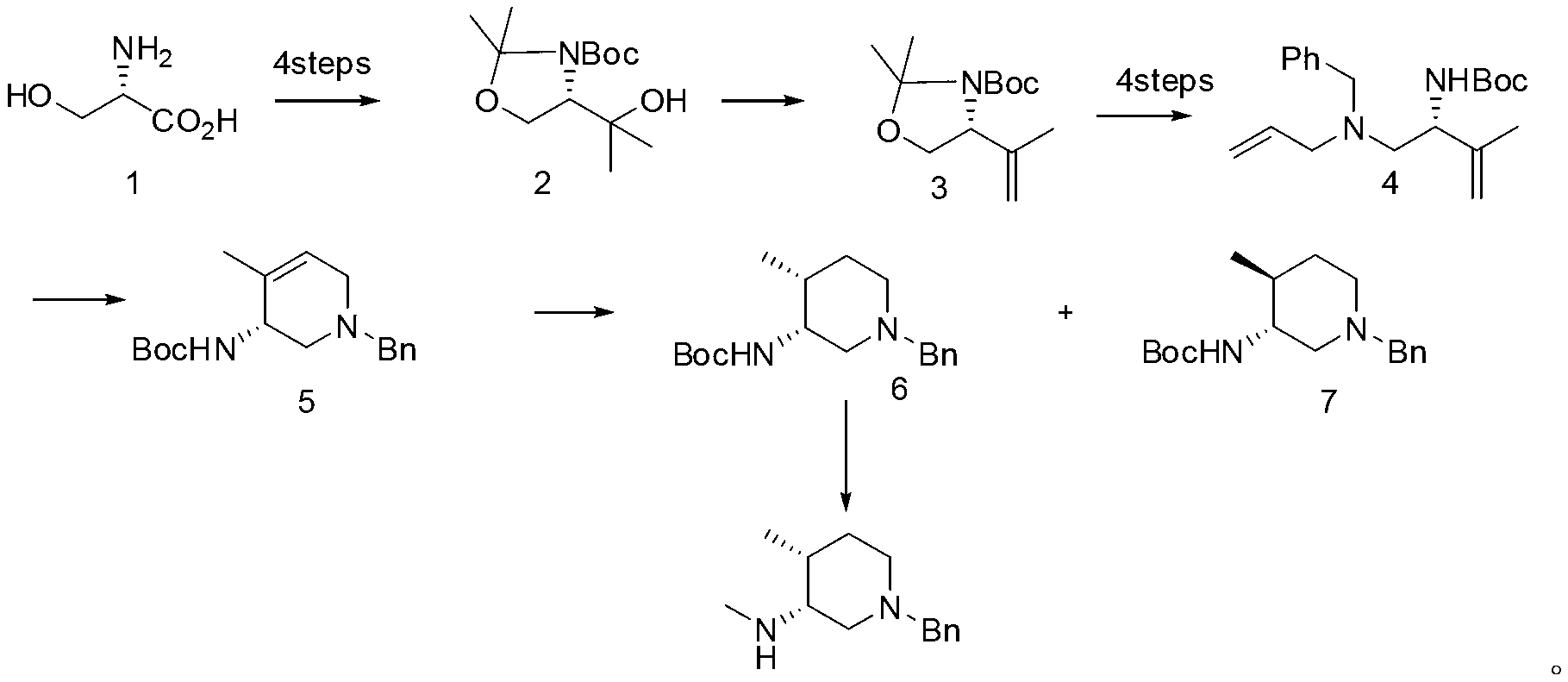
Synthesis method of (3R, 4R)-N-PG-4-methyl-3-methylaminopiperidine
A technology of -N-PG-4-, N-PG-2-, applied in the field of organic synthesis, can solve the problems of high price, expensive raw materials, high safety risk, etc., and achieve control of production cost, simple synthesis route and good reaction conditions mild effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The synthetic method of (3R, 4R)-N-Boc-4-methyl-3-methylaminopiperidine comprises the following steps:
[0038]1) Ring closure: 7.0g 2-butenal (0.1mol), 19.0g N-Boc-2-nitroethylamine (0.1mol), 0.0041g catalyst (0.00001mol, the molar amount is equivalent to 2-butenal 0.01%) and 0.0024g of benzoic acid (0.00002mol) were added to 50mL of water, reacted at 5°C for 0.5 hours, and then raised to room temperature (25°C) for 4 hours; after the reaction, add 0.1mL of trifluoroacetic acid to the system for dehydration, Continue to react for 3 hours at room temperature;
[0039] After the reaction is complete, add saturated sodium carbonate solution to the system to neutralize to a pH value of 6.0, extract with ethyl acetate, combine the extracts, dry over magnesium sulfate and remove the extraction solvent ethyl acetate to obtain (3R,4R)-N - Crude Boc-4-methyl-3-nitro-3,4-dihydropyridine. The crude product does not need to be further separated, and can be directly subjected to ...
Embodiment 2
[0045] The synthetic method of (3R, 4R)-N-Cbz-4-methyl-3-methylaminopiperidine comprises the following steps:
[0046] 1) Ring closure: take 7.0g 2-butenal (0.1mol), 22.4g N-Cbz-2-nitroethylamine (0.1mol), 0.41g catalyst (0.001mol), 0.24g o-chlorobenzoic acid (0.002mol ) into 50mL methanol, react at 0°C for 1 hour, then rise to room temperature (25°C) and react for 5 hours; after the reaction, add 0.1mL trifluoromethanesulfonic acid to the system for dehydration, and continue to react at room temperature for 3 hours;
[0047] After the reaction is completed, add saturated sodium bicarbonate solution to the system to neutralize to a pH value of 6.4, remove methanol and extract with ethyl acetate, combine the extracts, dry with magnesium sulfate, and remove the extraction solvent ethyl acetate to obtain (3R,4R)-N-Cbz--4-Methyl-3-nitro-3,4-dihydropyridine crude product.
[0048] 2) Hydrogenation reduction: Add the crude product obtained in step 1) and 0.5g catalyst (10%Pd / C) int...
Embodiment 3
[0052] The synthetic method of (3R, 4R)-N-Cbz-4-methyl-3-methylaminopiperidine comprises the following steps:
[0053] 1) Ring closure: 7.0g 2-butenal (0.1mol), 33.6g N-Cbz-2-nitroethylamine (0.15mol), 4.1g catalyst (0.01mol), 2.4g m-chlorobenzoic acid (0.02mol ) into ethanol, react at -10°C for 1 hour, then rise to 0°C for 5 hours; after the reaction, add 0.1mL trichloroacetic acid to the system for dehydration, and continue to react at 10°C for 3 hours;
[0054] After the reaction is complete, add saturated sodium phosphate solution to the system to neutralize to a pH value of 6.7, remove ethanol and extract with dichloromethane, combine the extracts, dry with sodium sulfate and remove the extraction solvent dichloromethane to obtain cis- (3R,4R)-N-Cbz--4-Methyl-3-nitro-3,4-dihydropyridine crude product.
[0055] 2) Hydrogenation reduction: dissolve the crude product obtained in step 1) in N,N-dimethylformamide, add 0.1g catalyst (10%Pt / C), hydrogen pressure 0.2MPa, and rea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com