Luminous metal-organic framework material with chemical sensing function as well as preparation method and application thereof
A metal-organic framework and chemical sensing technology, applied in luminescent materials, chemical instruments and methods, chemiluminescence/bioluminescence, etc., can solve the problems of expensive instruments, complicated pretreatment, and long time consumption, and achieve easy preparation and stability Good, simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
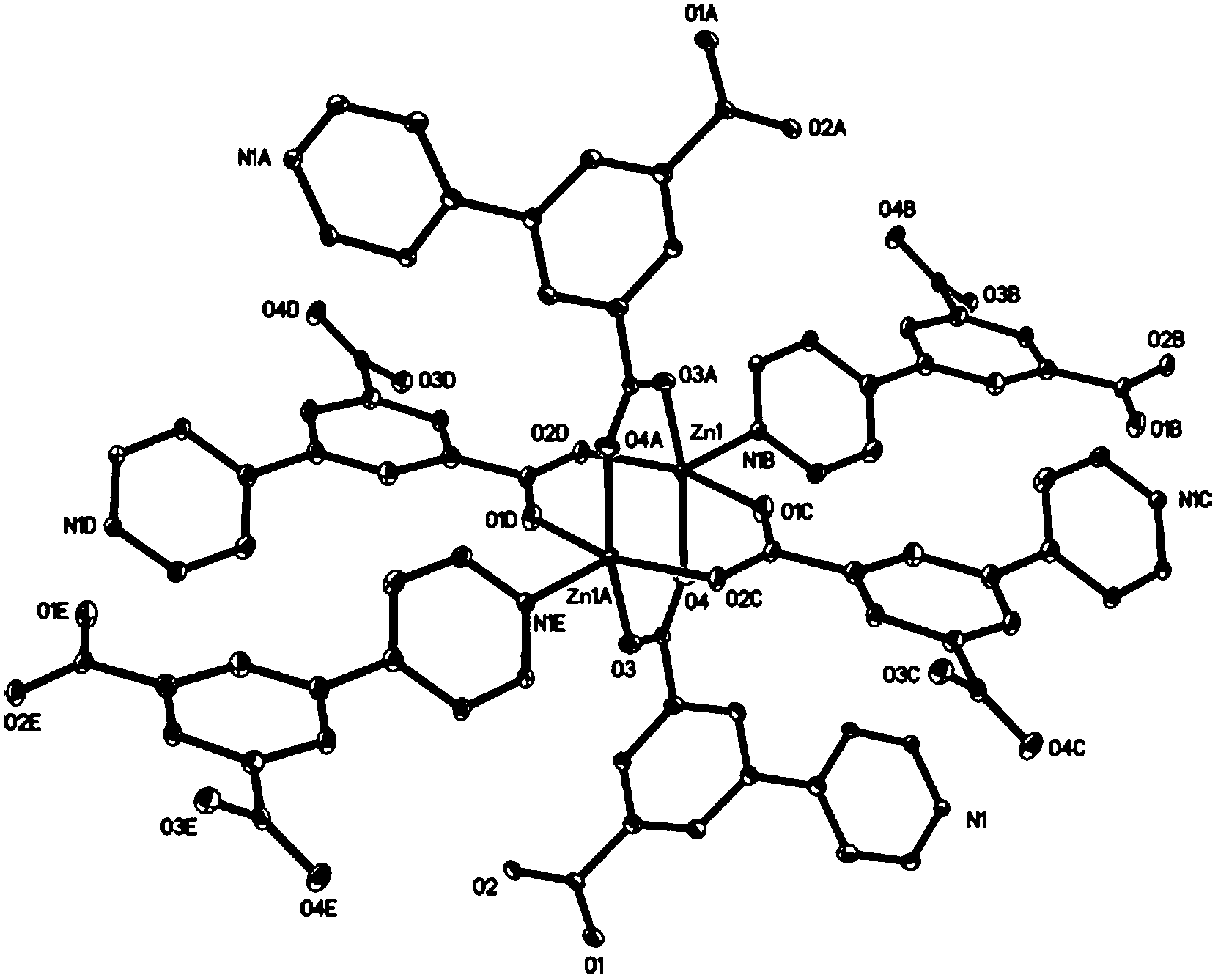
[0036] Synthesis of luminescent metal-organic framework materials: Weigh 29.8mg of zinc nitrate and 24.3mg of 5-(4-pyridyl)-1,3-benzenedicarboxylic acid and dissolve in 4.5ml of N,N-dimethylacetamide and 0.5ml of absolute ethanol The mixed solution was fully stirred, and then the mixed solution was transferred to a stainless steel reactor lined with polytetrafluoroethylene, crystallized in a synthesis oven at 100°C for 72 hours, and cooled to room temperature to obtain light yellow blocky crystals. The product was filtered, washed with N,N-dimethylacetamide, dried at room temperature, and collected. The yield was about 70%. The molecular structure of the compound is shown in figure 1 , the three-dimensional structure diagram of the compound is shown in figure 2 middle.
[0037] Such as image 3 with Figure 4 As shown, X-ray powder diffraction and thermogravimetric analysis show that the compound is of high purity, and its skeleton structure can be stabilized to 380° C. u...
Embodiment 2
[0039]Synthesis of luminescent metal-organic framework materials: Weigh 59.6mg of zinc nitrate and 24.3mg of 5-(4-pyridyl)-1,3-benzenedicarboxylic acid dissolved in 5ml of N,N-dimethylacetamide solution, stir well, and then The mixed solution was transferred into a stainless steel reactor lined with polytetrafluoroethylene, crystallized in a synthesis oven at 90°C for 72 hours, and cooled to room temperature to obtain light yellow blocky crystals. The product was filtered, washed with N,N-dimethylacetamide, dried at room temperature, and collected. The yield was about 67%.
Embodiment 3
[0041] Synthesis of luminescent metal-organic framework materials: Weigh 13.6mg of zinc chloride and 24.3mg of 5-(4-pyridyl)-1,3-benzenedicarboxylic acid and dissolve in 4.5ml of N,N-dimethylacetamide and 0.5ml of anhydrous The mixed solution of ethanol was stirred thoroughly, and then the mixed solution was transferred into a stainless steel reactor lined with polytetrafluoroethylene, crystallized in a synthesis oven at 90°C for 48 hours, cooled to room temperature, and light yellow blocky crystals were obtained. The product was filtered, washed with N,N-dimethylacetamide, dried at room temperature, and collected. The yield was about 72%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com