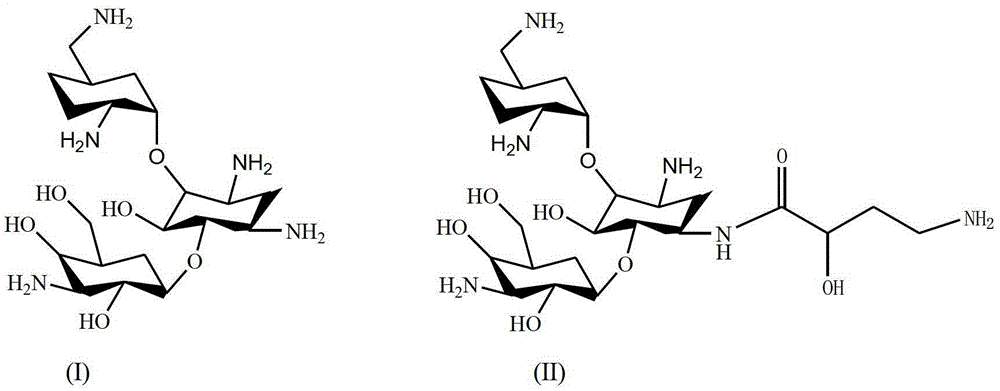
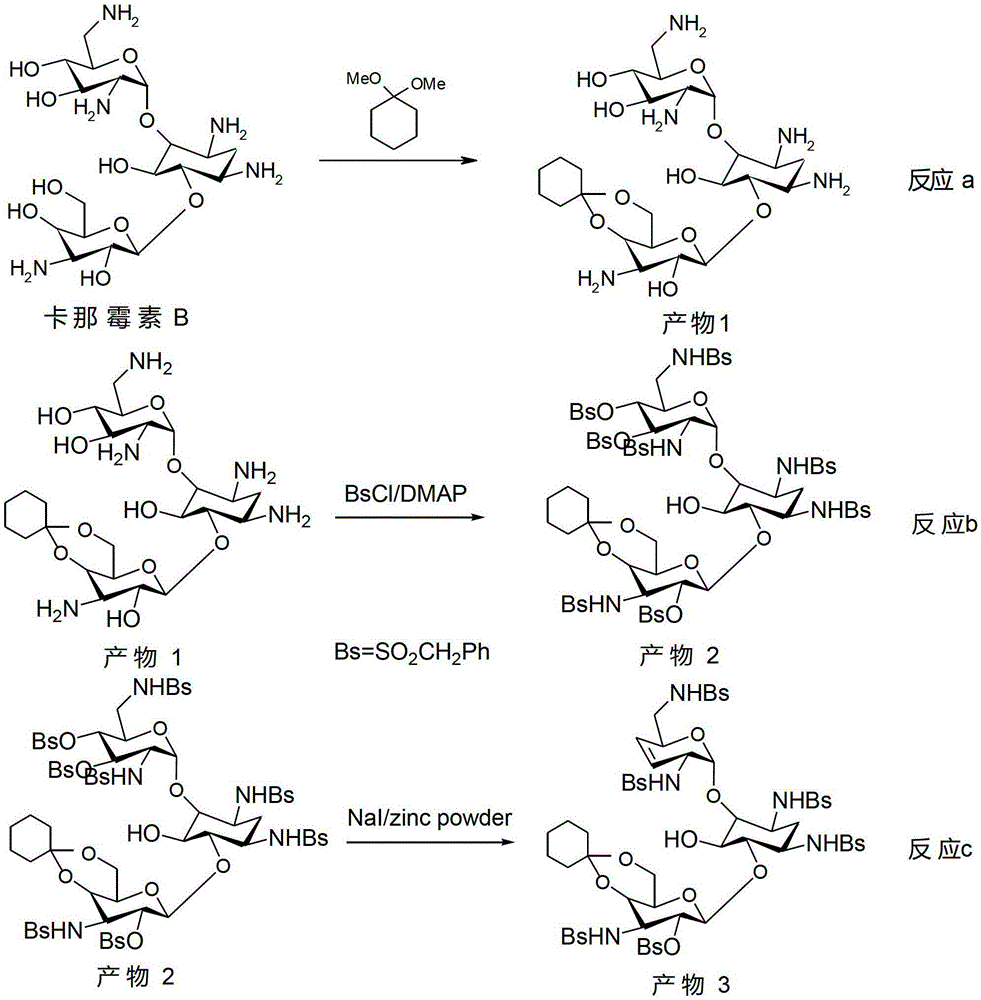
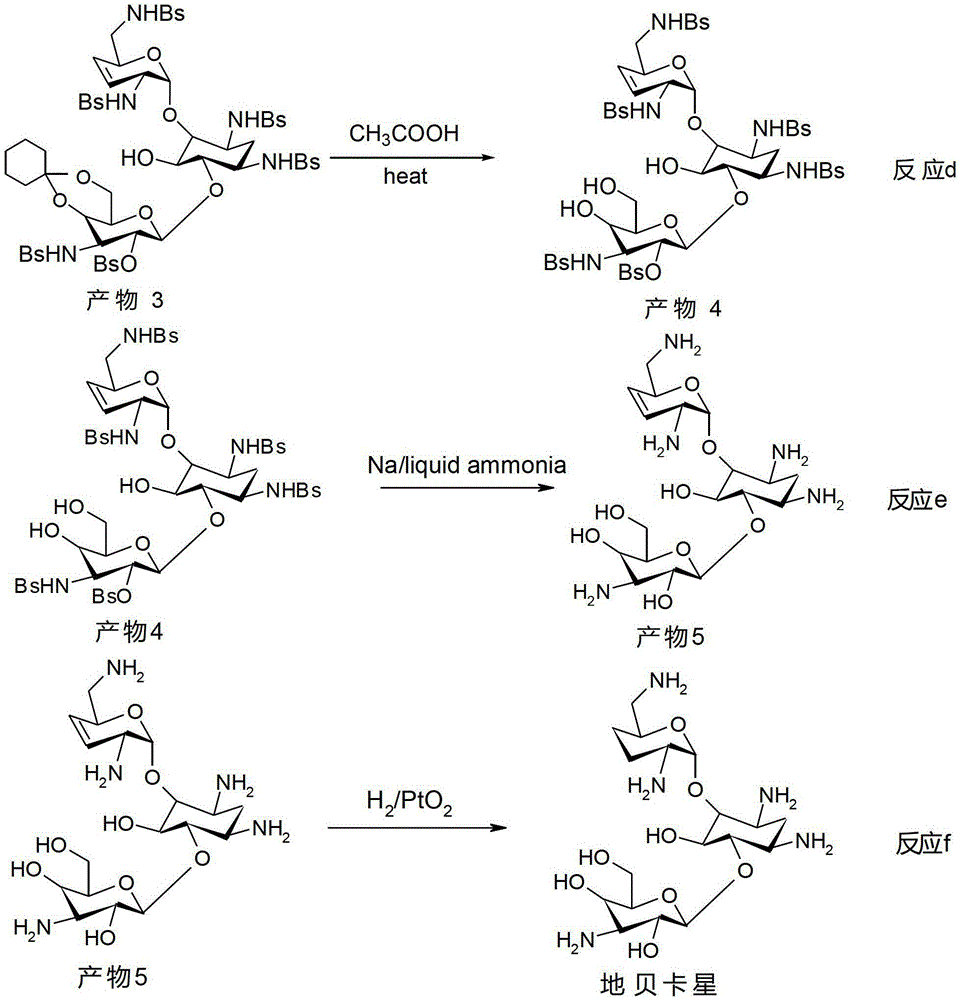
The synthetic method of dibekacin and arbekacin
A synthesis method and technology of dibekacin are applied in the field of synthesis of dibekacin and arbekacin, which can solve the problems of long process route, health hazards of operators, difficulty in enlarging production, etc., so as to reduce environmental pollution and shorten production time. Three-step reaction, the effect of shortening the production cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] (1) Weigh 9.66g (20mmol) of Kanamycin B and 10.6g (100mmol) of anhydrous sodium carbonate, dissolve them in 50mL of water, add 50mL of isopropanol, and weigh di-tert-butyl dicarbonate 26.2g (120mmol) was added to it, reacted at 30°C for 6 hours, filtered, and the filtrate was collected to obtain 1,3,2',6',3''-penta-nitrogen-tert-butoxycarbonyl-kanamycin B18.3g, yield 93%.
[0076] (2) Weigh 1,3,2′,6′,3′′-penta-nitrogen-tert-butoxycarbonyl-kanamycin B18.3g (18.6mmol), anhydrous p-toluenesulfonic acid 0.64g (3.7 mmol), dissolve it in 100mL N,N-dimethylformamide, add 7.7mL (55.8mmol) of 1,1-dimethoxycyclohexane, react at 40°C for 12 hours, stop the reaction, and solution was poured into 1L of water to disperse, filtered, and the filtrate was collected to obtain 1,3,2′,6′,3′′-penta-nitrogen-tert-butoxycarbonyl-4″,6″-oxygen-cyclohexylene- Kanamycin B18.2g, yield 92%.
[0077] (3) Weigh 18.2g (17.1mmol ), dissolved it in 200mL toluene, added 11.4g (25.6mmol) of 2,4,5-trii...
Embodiment 2
[0086] In step (1), the mol ratio of di-tert-butyl dicarbonate, sodium carbonate and kanamycin B is 5:5:1 (i.e. di-tert-butyl dicarbonate 21.8g, sodium carbonate 10.6g, kanamycin B Mycin B9.66g), other steps are with embodiment 1, obtain 1,3,2',6',3''-five-aza-tert-butoxycarbonyl-kanamycin B16.1g, yield is 82 %.
Embodiment 3
[0088] In step (1), the mol ratio of di-tert-butyl dicarbonate, sodium carbonate and kanamycin B is 10:5:1 (i.e. di-tert-butyl dicarbonate 43.6g, sodium carbonate 10.6g, kanamycin B Mycin B9.66g), other steps are with embodiment 1, obtain 1,3,2',6',3''-five-aza-tert-butoxycarbonyl-kanamycin B18.5g, yield is 94 %.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com