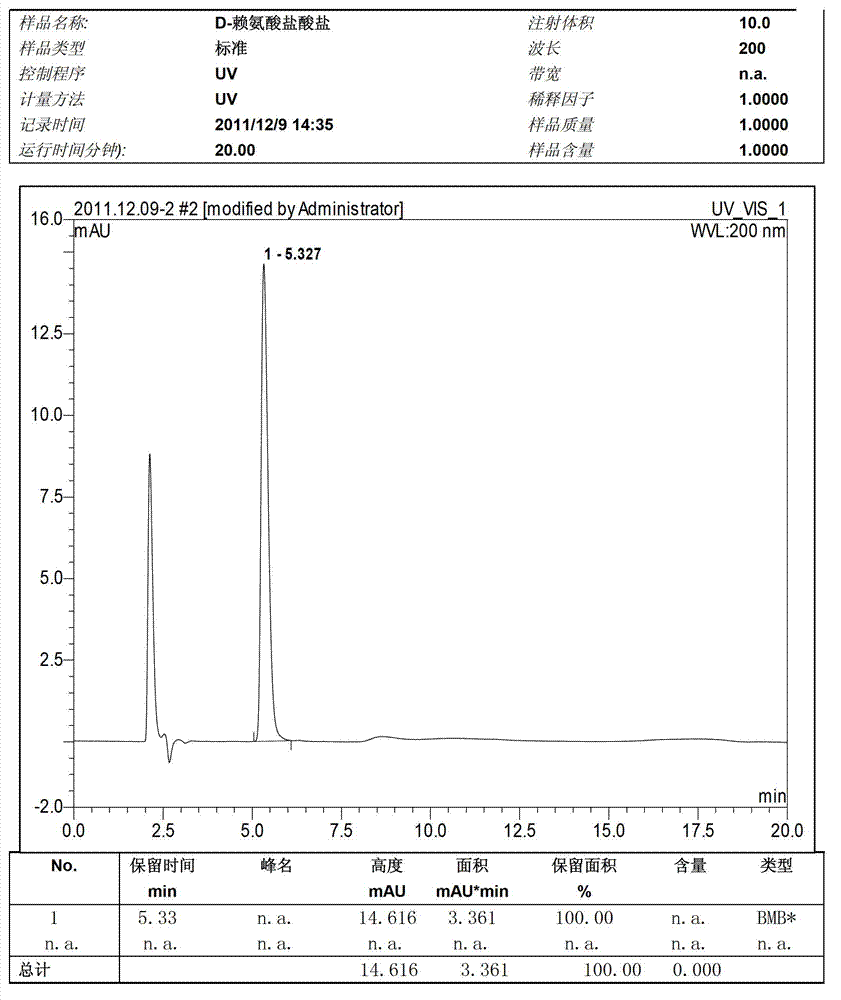
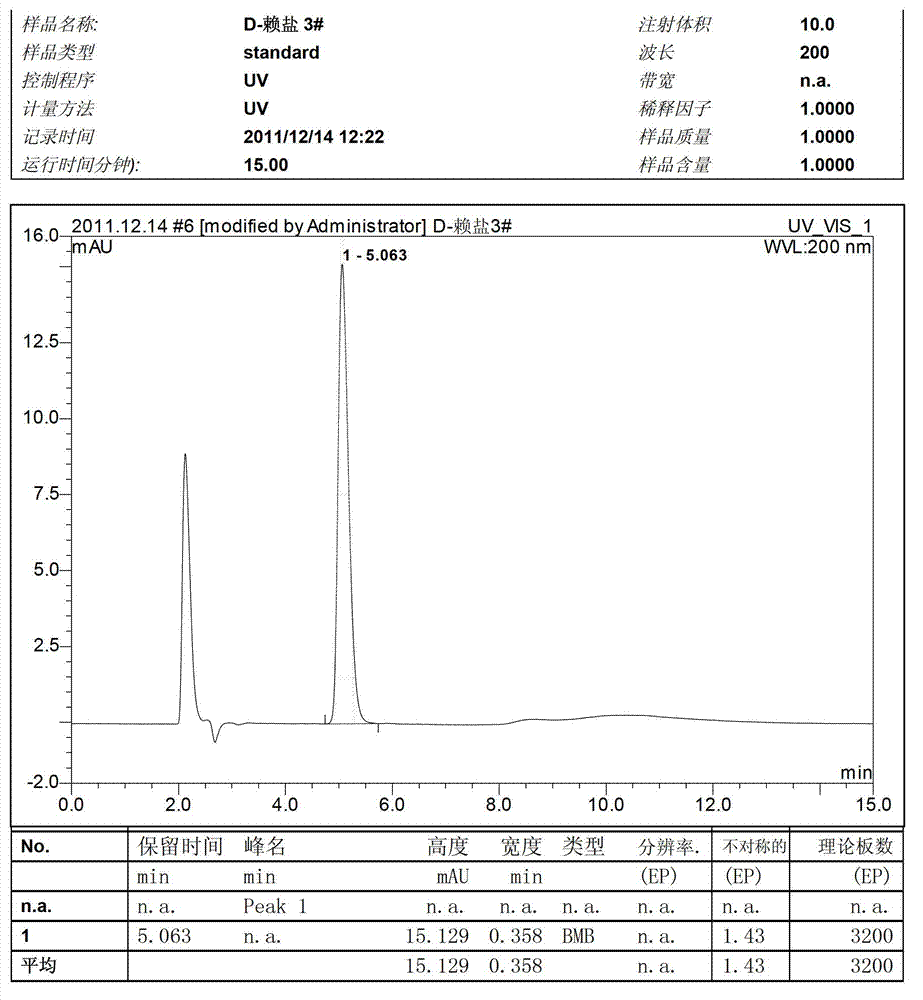
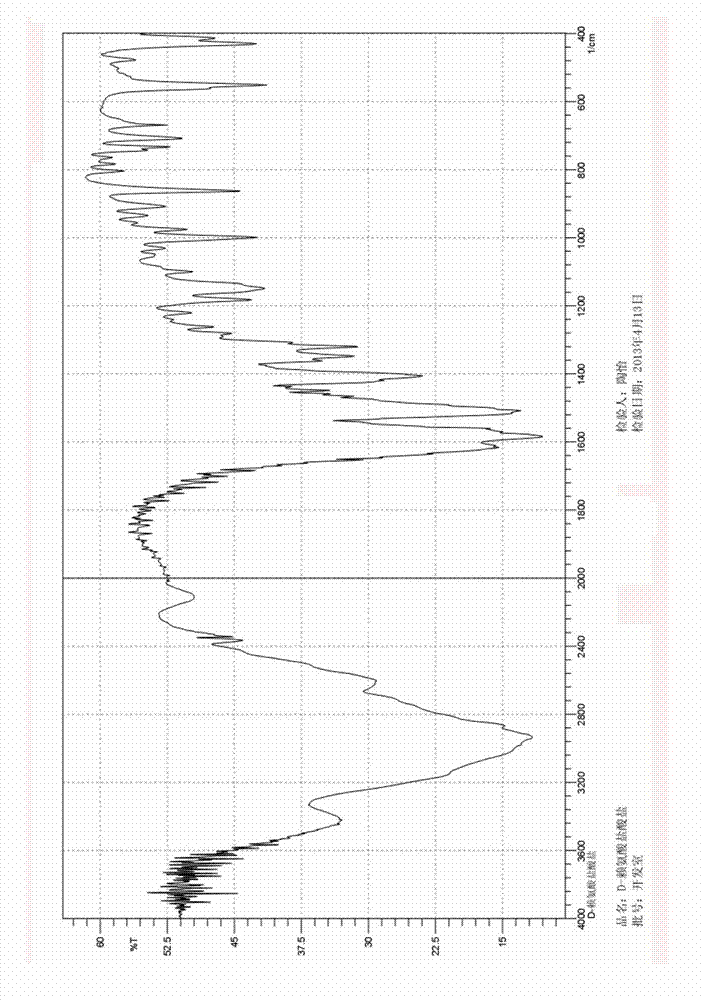
Preparation method of D-lysine hydrochloride
A technology of lysine hydrochloride and lysine, which is applied in the preparation of organic compounds, chemical instruments and methods, and cyanide reaction preparation, etc., can solve the problems of no contribution to environmental protection, achieve stable product quality, save concentration Energy consumption, good separation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] A preparation method of D-lysine hydrochloride, which comprises the following steps carried out in sequence:
[0044] (1) Take 140mL of DL-lysine solution equivalent to 100g of DL-lysine, add it to a 500mL three-necked flask, heat up to 50℃~60℃ under stirring, then add 75gL-tartaric acid to react to form diastereomers Precipitation of salt L-tartrate D-lysine;
[0045] (2) Filter and separate the precipitate and solution of the diastereomeric salt L-tartrate D-lysine, collect 53.75 g of the diastereomeric salt L-tartrate D-lysine, and set aside;
[0046] (3) Add 25~55g of water to wash the diastereomeric salt L-tartrate D-lysine, filter dry, then add 120g methanol, then wash the diastereomeric L-tartrate D-lysine salt, and then filter Dried to obtain 45g of diastereomer L-tartrate D-lysine salt, set aside;
[0047] (4) Dissolve the diastereomeric salt L-tartrate D-lysine with 1~2 times the mass of water, and then add 13.5g of calcium hydroxide under stirring at room t...
Embodiment 2
[0058] A preparation method of D-lysine hydrochloride, which comprises the following steps carried out in sequence:
[0059] (1) Take 590mL of DL-lysine solution equivalent to 500g of DL-lysine, add it into a 1000mL three-necked flask, heat up to 40°C-50°C under stirring, then add 360gL-tartaric acid to react to form diastereomers Precipitation of salt L-tartrate D-lysine;
[0060] (2) Filter and separate the diastereomeric salt L-tartrate D-lysine precipitate and solution, collect 269.5 g of the diastereomeric salt L-tartrate D-lysine, and set aside;
[0061] (3) Add a proportional amount of water to wash the diastereomer salt L-tartrate D-lysine, filter to dry, then add a proportional amount of methanol, then wash the diastereomer L-tartrate D-lysine salt, and then filter Dried to obtain 226.4g of diastereomer L-tartrate D-lysine salt, set aside;
[0062] (4) Dissolve 226.4g of the diastereomeric salt L-tartrate D-lysine in 2~3 times the mass of water, and then add 78g of ...
Embodiment 3
[0072] A preparation method of D-lysine hydrochloride, which comprises the following steps carried out in sequence:
[0073] (1) Take 1500mL of DL-lysine solution equivalent to 1000g of DL-lysine, add it to a 3000mL three-necked flask, heat up to 30-70°C under stirring, then add 750gL-tartaric acid to react to form diastereomeric salts L-tartrate D-lysine precipitation;
[0074] (2) Filter and separate the precipitate and solution of the diastereomeric salt L-tartrate D-lysine, collect 540 g of the diastereomeric salt L-tartrate D-lysine, and set aside;
[0075] (3) Add 280g of water to wash the diastereomeric salt L-tartrate D-lysine, filter dry, then add 1200g methanol, then wash the diastereomeric L-tartrate D-lysine salt, then filter dry, Obtain 440.7g of diastereomer L-tartrate D-lysine salt, set aside;
[0076] (4) Dissolve 440.7g of the diastereomeric salt L-tartrate D-lysine in 1 to 3 times the mass of water, then add 85g of calcium hydroxide and 70g of calcium carbo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com