Synthetic method of pentacycloundecane
A synthetic method and eleven technology, applied in the field of synthesis of high-density clathrate strained ring hydrocarbon - pentacycloundecane (PCUD), which can solve the harsh conditions required for synthesis, limit the use of PCUD, and not suitable for Amplify production and other issues to achieve the effects of reducing synthesis costs, increasing operability and safety, and saving manpower
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
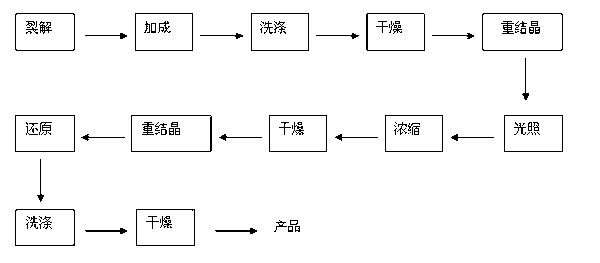
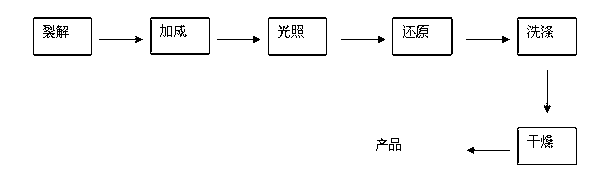
Image
Examples
Embodiment 1
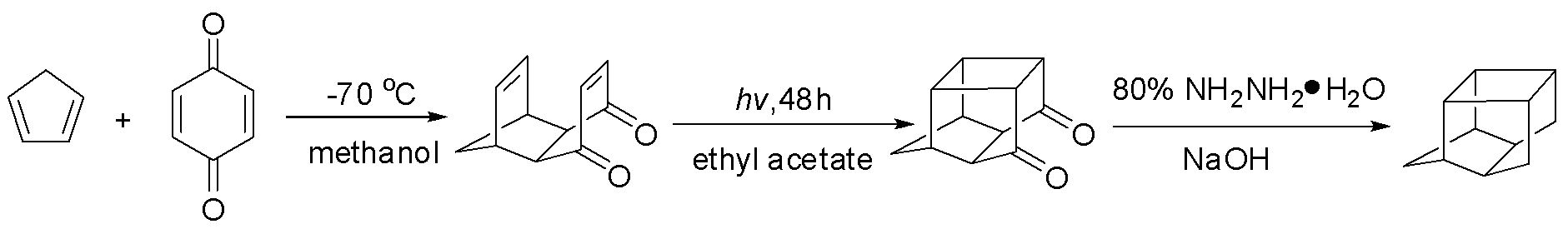
[0028] Tricycloundecanedienedione (C 11 h 8 o 2 )Synthesis:
[0029] Diels-Alder addition reaction of cyclopentadiene and benzoquinone: add benzoquinone (2690g, 24.88mol) and 15L of dipropylene glycol in a 25L jacketed kettle, add cyclopentadiene (1860g, 27.3mol) dropwise, Stir and control the temperature of the solution at 0-5°C. After the dropwise addition, the reaction was carried out for 1 hour, and the temperature of the reaction system was slowly raised to room temperature. After the stirring was stopped, a brownish-yellow solution appeared in the reaction kettle.
[0030] Pentacycloundecanedione (C 11 h 8 o 2 )Synthesis:
[0031] The above solution was put into a quartz glass tube with a diameter of 2.5 cm, and irradiated with ultraviolet light for 25 hours to obtain a dipropylene glycol transparent solution containing pentacycloundecanedione, with a chromatographic yield of 92%.
[0032] Pentacycloundecane (C 11 h 14 , PCUD) synthesis:
[0033] Put the dipro...
Embodiment 2
[0036]Tricycloundecanedienedione (C 11 h 8 o 2 )Synthesis:
[0037] Diels-Alder addition reaction of cyclopentadiene and benzoquinone: add benzoquinone (2690g, 24.88mol) and 15L of diethylene glycol in a 25L jacketed kettle, add cyclopentadiene (1860g, 27.3mol) dropwise , stir, and control the temperature of the solution at 5-10°C. After the dropwise addition, the reaction was carried out for 1 hour, and the temperature of the reaction system was slowly raised to room temperature. After the stirring was stopped, a brownish-yellow solution appeared in the reaction kettle.
[0038] Pentacycloundecanedione (C 11 h 8 o 2 )Synthesis:
[0039] The above solution was put into a quartz glass tube with a diameter of 2.5 cm, and irradiated with ultraviolet light for 22 hours to obtain a diethylene glycol transparent solution containing pentacycloundecanedione, with a chromatographic yield of 90%.
[0040] Pentacycloundecane (C 11 h 14 , PCUD) synthesis:
[0041] Put the dieth...
Embodiment 3
[0043] Tricycloundecanedienedione (C 11 h 8 o 2 )Synthesis:
[0044] Diels-Alder addition reaction of cyclopentadiene and benzoquinone: add benzoquinone (2160g, 20mol) and 12L of dipropylene glycol in a 25L jacketed kettle, add cyclopentadiene (1498g, 22.0mol) dropwise, and stir , the temperature of the solution is controlled at 0-5°C. After the dropwise addition, the reaction was carried out for 1 hour, and the temperature of the reaction system was slowly raised to room temperature. After the stirring was stopped, a brownish-yellow solution appeared in the reaction kettle.
[0045] Pentacycloundecanedione (C 11 h 8 o 2 )Synthesis:
[0046] The above solution was put into a quartz glass tube with a diameter of 2.5 cm, and irradiated with ultraviolet light for 30 hours to obtain a dipropylene glycol transparent solution containing pentacycloundecanedione, with a chromatographic yield of 93%.
[0047] Pentacycloundecane (C 11 h 14 , PCUD) synthesis:
[0048] Put the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
| heating value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com