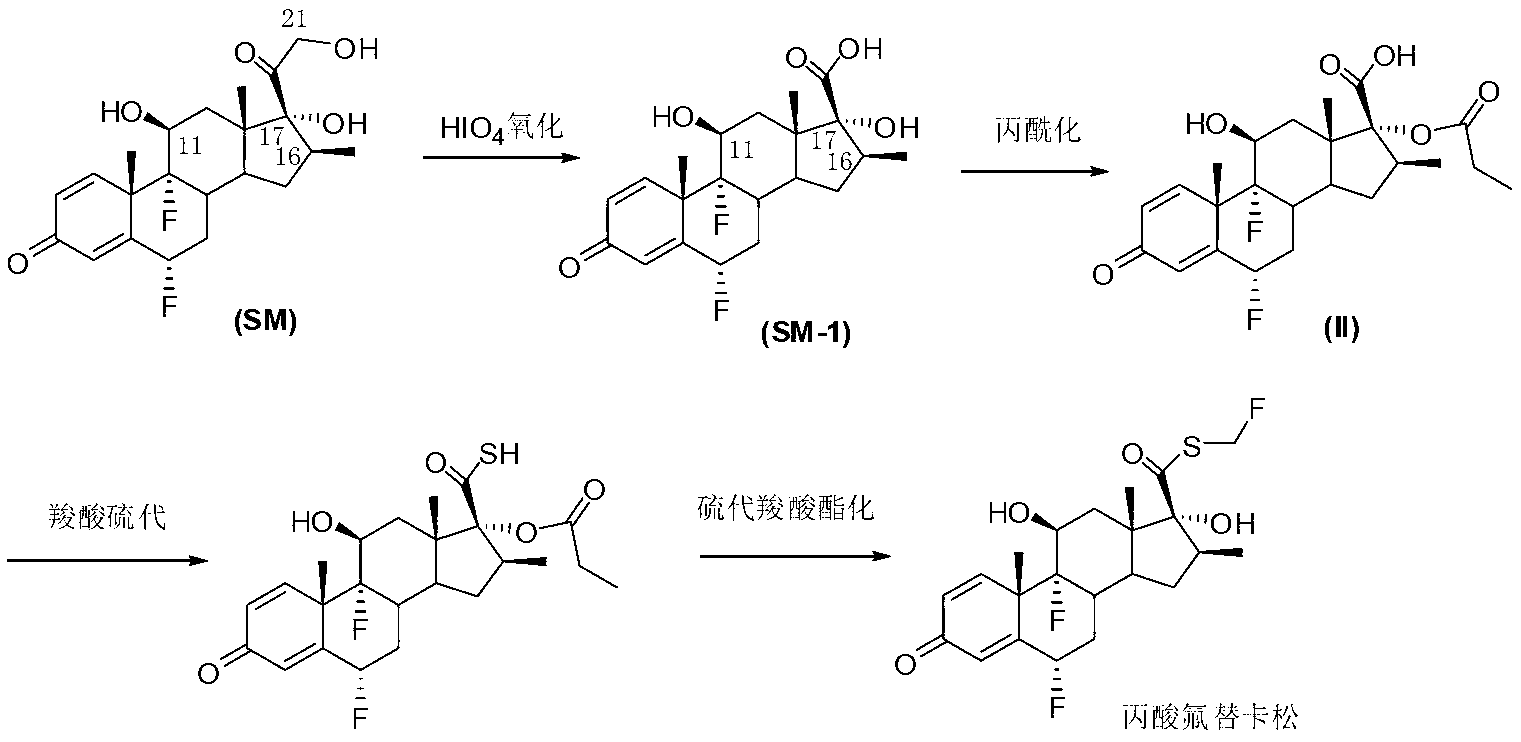
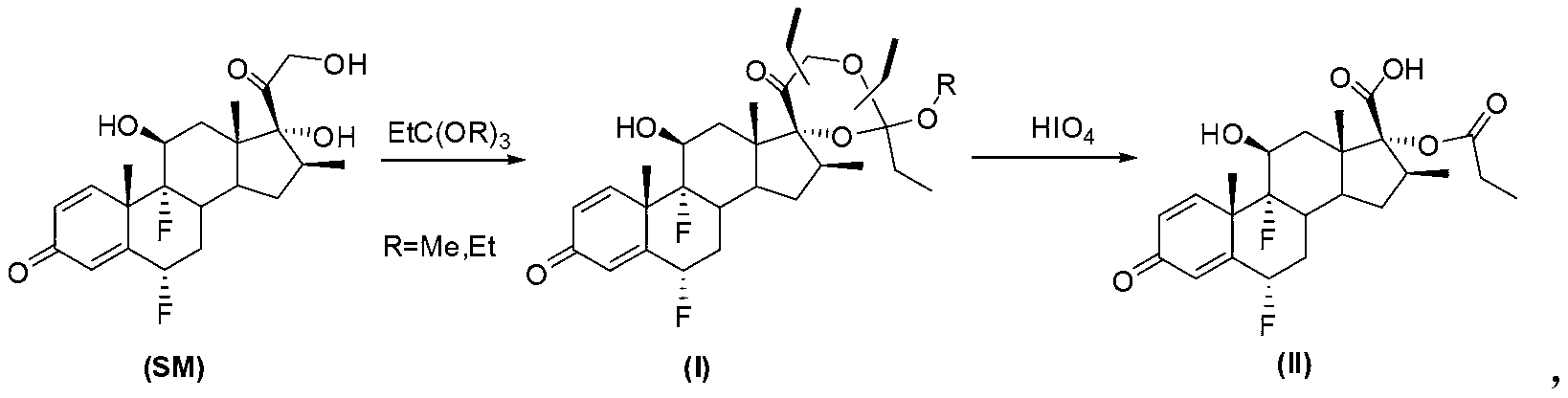
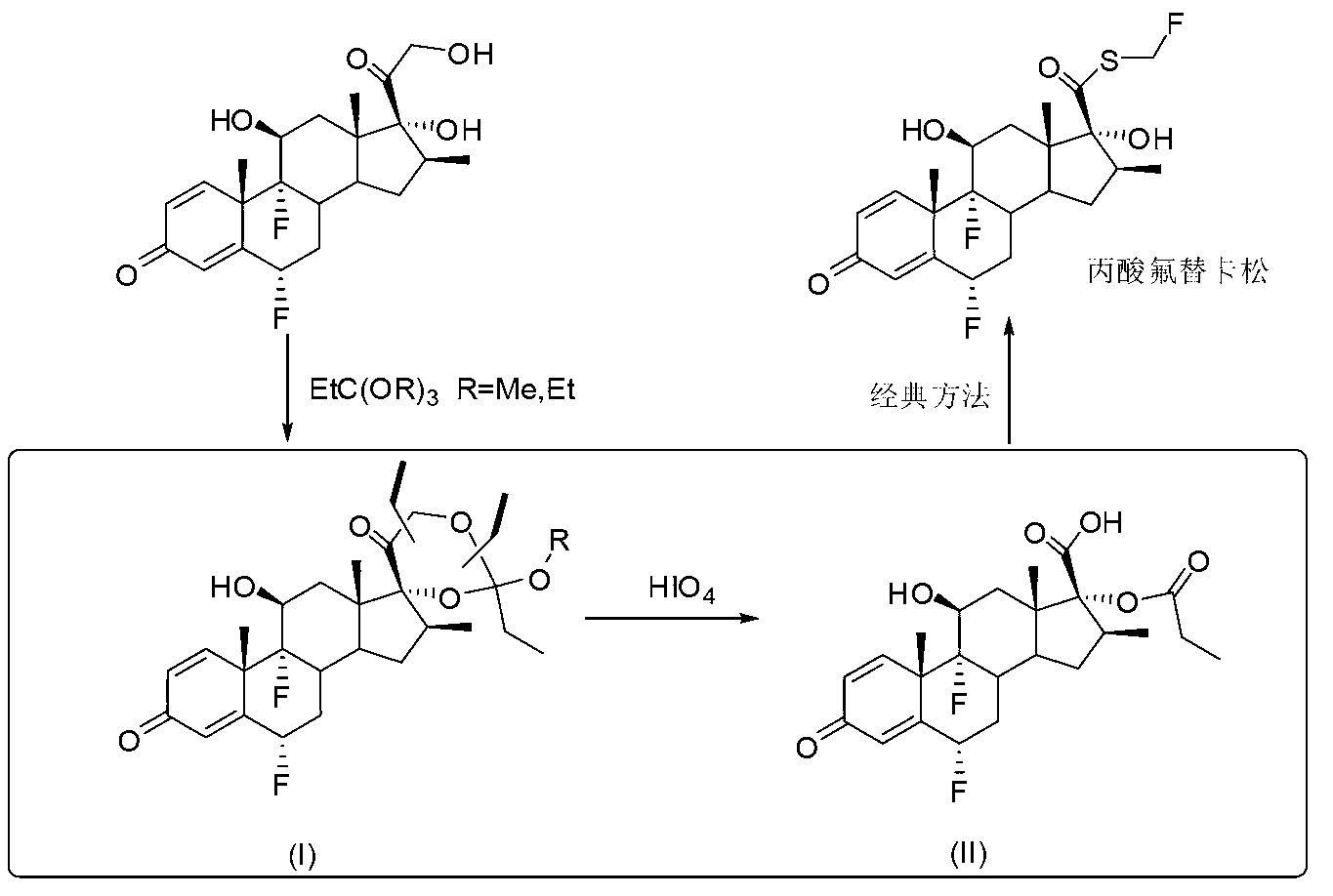
Method for synthesizing key intermediate of fluticasone propionate
A technology of fluticasone propionate and a synthesis method, which is applied in the directions of steroids, bulk chemical production, organic chemistry, etc., can solve the problems of reduced yield, slowed reaction speed, etc., and achieves improved purity, simplified post-treatment, and process cost. improved effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] The synthesis of embodiment 1 flumetasone ethyl cyclopropionate I-1
[0022]
[0023] Suspend 4.2g of flumetasone (SM) in 20mL of dichloromethane, add 4.2ml of triethyl orthopropionate, add 210mg of p-toluenesulfonic acid at room temperature, stir for 5 minutes and the system is completely clear, and TLC shows that the reaction is basically complete. To ensure complete reaction, the reaction was continued for 30 minutes. Remove most of the solvent to obtain a viscous oil, add 100mL of petroleum ether, stir at room temperature, the solid gradually precipitates, filter with suction, wash twice with petroleum ether (25mL), and drain the obtained solid I-1 to obtain 4.1g of pure product .
[0024] 1 H NMR (400MHz, CDCl 3 )δ7.27(d,J=9.9Hz,1H),6.32(dd,J=10.1,1.7Hz,1H),6.10(s,1H),4.35(dd,J=9.3,5.0Hz,1H), 4.00(d,J=17.2Hz,1H),3.89(d,J=16.7Hz,1H),3.47(dq,J=9.2,7.3Hz,1H),3.35(dq,J=8.8,7.1Hz,1H ),1.54(s,3H),1.11(t,J=7.0Hz,3H),0.97(s,3H),0.95(t,J=9.2Hz,3H),0.92(d,J=7.2Hz,3H ...
Embodiment 2
[0025] The synthesis of embodiment 2 flumetasone methyl cypionate I-2
[0026]
[0027] Use trimethyl orthopropionate to replace triethyl orthopropionate in Example 1, the following cyclic ortho ester intermediate I-2 can be obtained in the same way:
[0028] Suspend 4.2g of flumetasone (SM) in 20mL of dichloromethane, add 4.2ml of trimethyl orthopropionate, and add 210mg of p-toluenesulfonic acid at room temperature. After stirring for 5 minutes, the system is completely clear, and TLC shows that the reaction is basically complete. To ensure complete reaction, the reaction was continued for 30 minutes. Remove most of the solvent to obtain a viscous oil, add 100mL of petroleum ether, stir at room temperature, the solid gradually precipitates, filter with suction, wash twice with petroleum ether (25mL), and drain the obtained solid I-2 to obtain 4.1g of pure product .
[0029] 1 H NMR (400MHz, CDCl 3 )δ7.25(d,J=9.7Hz,1H),6.32(dd,J=9.9,1.7Hz,1H),6.14(s,1H),4.38(dd,J=9.5,5...
Embodiment 3
[0030] The oxidative hydrolysis of embodiment 3 flumetasone ethyl cyclopropionate I-1
[0031]
[0032] Dissolve 2.4 g of flumetasone ethyl cypionate I-1 in 50 mL of chloroform, add 100 mL of water and 5 mL of acetone, add 1.0 g of periodic acid in 20 mL of aqueous solution dropwise in an ice bath, add one hour, and continue stirring for 30 minutes. TLC tracking showed that the reaction was complete, separated, the oil phase was washed twice with 50 ml of water, dried over anhydrous sodium sulfate, concentrated to semi-dry, added 20 ml of petroleum ether, stirred for 10 minutes, collected the precipitated solid, and drained to obtain the pure intermediate II2. 2 grams. The crude product after washing and drying can also be directly used in the next step.
[0033] Compound II: molecular weight, 452.49; 1 H NMR (400MHz, CDCl 3 )δ7.27(t,J=5.0Hz,1H),6.28(dd,J=10.1,1.4Hz,1H),6.03(s,1H),4.52(s,1H),4.29(dd,J=24.7 ,4.9Hz,1H),3.12(t,J=4.8Hz,1H),2.91–2.70(m,1H),2.59(td,J=13.4,4.5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com