Method for relative quantitative analysis of protein phosphorylation modification level
A quantitative analysis and phosphorylation technology, applied in the field of analysis, can solve the problems of large labeling reagents, increased experimental cost and complexity, consumption, etc., to achieve the effect of improving sensitivity and accuracy, reducing experimental cost, and reducing operational complexity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0028] The following examples can enable those skilled in the art to understand the present invention more fully, but do not limit the present invention in any way.
[0029] Experimental samples: mutant (mut) and wild type (wt) of a certain protein kinase
[0030] experimental method:
[0031] 1. The two experimental samples mut and wt were digested with trypsin to convert the protein into a polypeptide mixture.
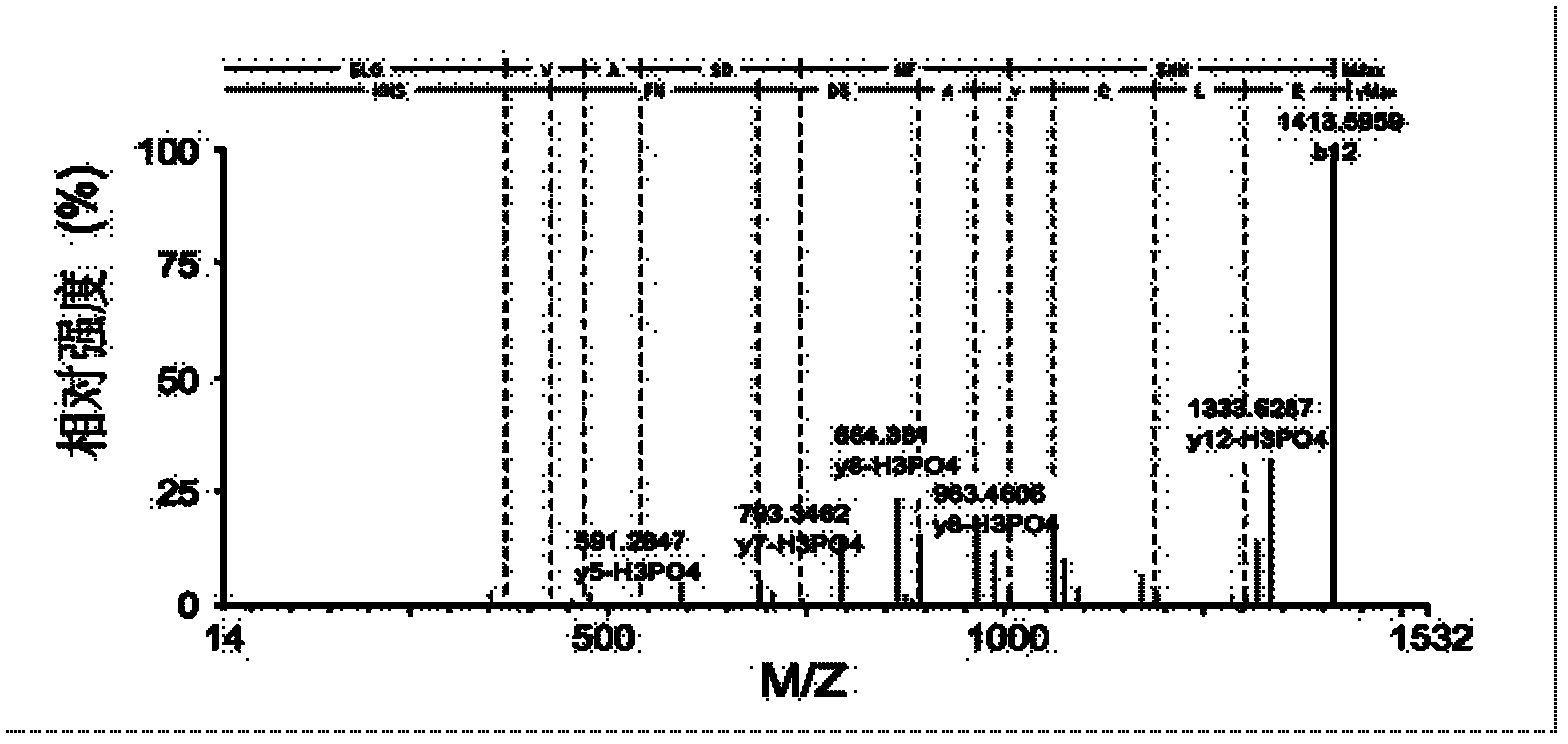
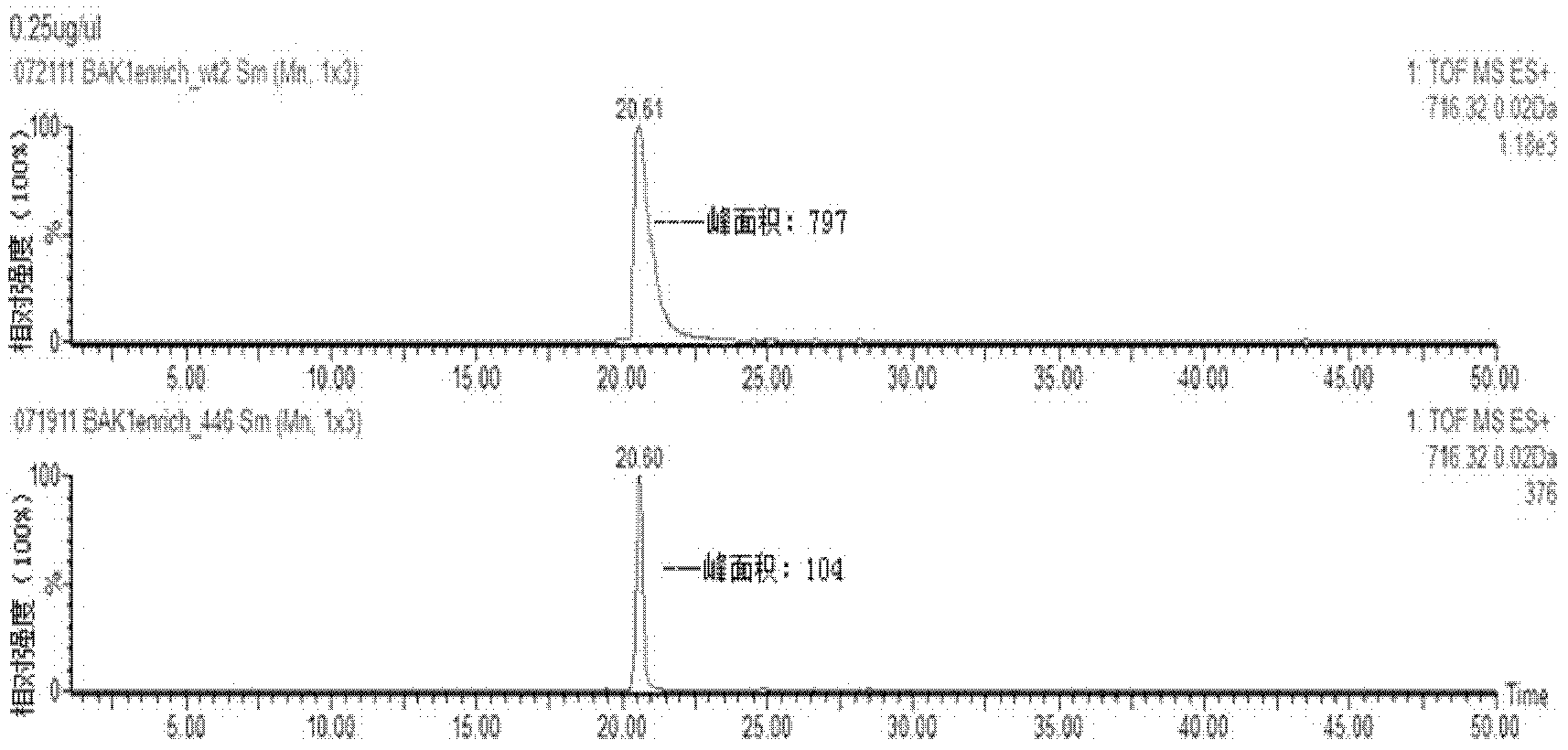
[0032] 2. Add an appropriate amount of internal standard substance (enzymatic hydrolysis product of β-casein) to the enzymatic hydrolysis samples of the two proteins to enrich the phosphorylated peptides together.
[0033] 3. Equilibrate the titanium dioxide affinity column with solution A (80% acetonitrile, 0.4% trifluoroacetic acid) and solution B (25% lactic acid, 60% acetonitrile, 0.3% trifluoroacetic acid) respectively, then mix the enzymolysis sample with Solution B is loaded onto the affinity column together. During centrifugation, the phosphorylated peptide...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com