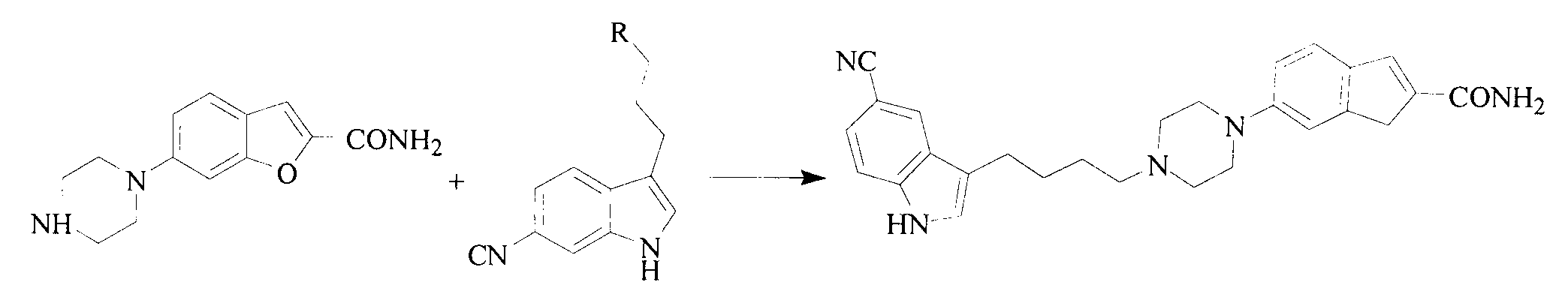
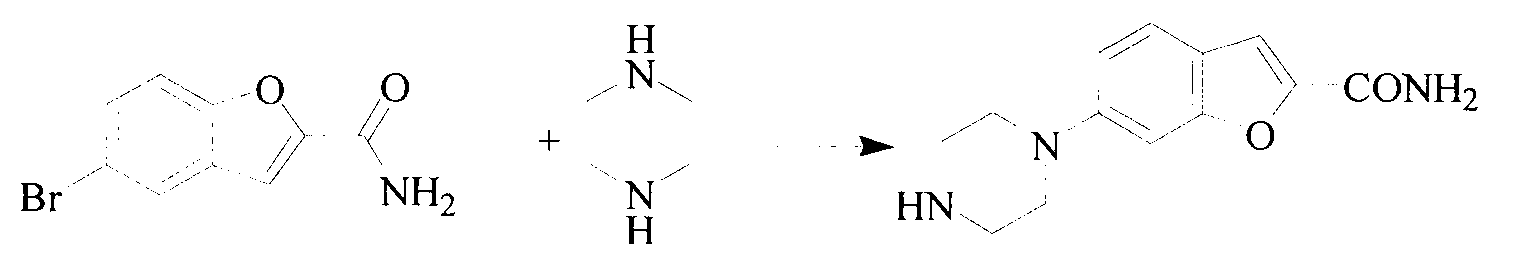
Method for preparing vilazodone hydrochloride midbody
A technology for vilazodone hydrochloride and intermediates, which is applied in the field of preparation of vilazodone hydrochloride intermediates, can solve problems such as complicated operation, high cost, and long reaction steps, and achieve high reaction yield, low cost, and easy operation. simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Preparation of compound II
[0027] Add bis(2-chloroethyl)amine hydrochloride (1908g, 13.20mol) and compound III (1500g, 7.32mol) to a 20L reaction flask successively, add 15L chlorobenzene and 1L cyclohexanol and stir to dissolve, then Add tetrabutylammonium bromide (1170g, 3.6mol), heat up to 130°C, reflux for 60h, monitor by TLC (dichloromethane:methanol:ammonia=85:10:5) After the reaction compound III basically disappears, stop Heat and cool to room temperature. filter. The filter cake was washed twice with ethanol. Air drying at 45°C gave 1602g of compound II, yield: 85%. h 1 -NMR (DMSO, 500MHz) δ: 1.28-1.30(t, 3H), 2.86-2.89(t, 4H), 3.02-3.04(t, 4H), 3.24(s, 2H), 4.73-4.78(q, 4H ), 7.14-7.16 (t, 3H), 7.40 (s, 1H), 7.44-7.48 (m, 2H), 7.58 (s, 1H), 8.01 (s, 1H).
Embodiment 2
[0029] Preparation of compound I
[0030] Compound II (200 g, 0.65 mol) was added to a 5 L autoclave, followed by 1.5 L of isopropanol. Introduce ammonia gas, pressurize to 0.15Mpa, and react at 10°C for 25h. Monitored by TLC (dichloromethane:methanol:ammonia water=85:10:5), the reaction compound II basically disappeared. The reaction was stopped, the solid was filtered off, and the solid was washed twice with methanol. 121.5 g of compound I was obtained by blast drying at 40° C., with a yield of 76.9%. h 1 -NMR (DMSO, 500MHz) δ: 2.86-2.89(t, 4H), 3.02-3.04(t, 4H), 3.24(s, 2H), 7.14-7.16(t, 3H), 7.40(s, 1H), 7.44-7.48 (m, 2H), 7.58 (s, 1H), 8.01 (s, 1H).
Embodiment 3
[0032] Preparation of compound I
[0033] Compound II (200 g, 0.65 mol) was added to a 5 L autoclave, followed by 1.5 L of ethanol. Ammonia gas was introduced, and the reaction was carried out under normal pressure at 25°C for 30h. Monitored by TLC (dichloromethane:methanol:ammonia=85:10:5) the reaction compound II disappeared. The reaction was stopped and the solid was filtered off. The solid was washed 2 times with methanol. Air drying at 40°C yielded 92.6 g of compound I with a yield of 78.6%. h 1 -NMR (DMSO, 500MHz) δ: 2.85-2.87(t, 4H), 3.02-3.05(t, 4H), 3.23(s, 2H), 7.14-7.17(t, 3H), 7.40(s, 1H), 7.44-7.49 (m, 2H), 7.58 (s, 1H), 8.01 (s, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com