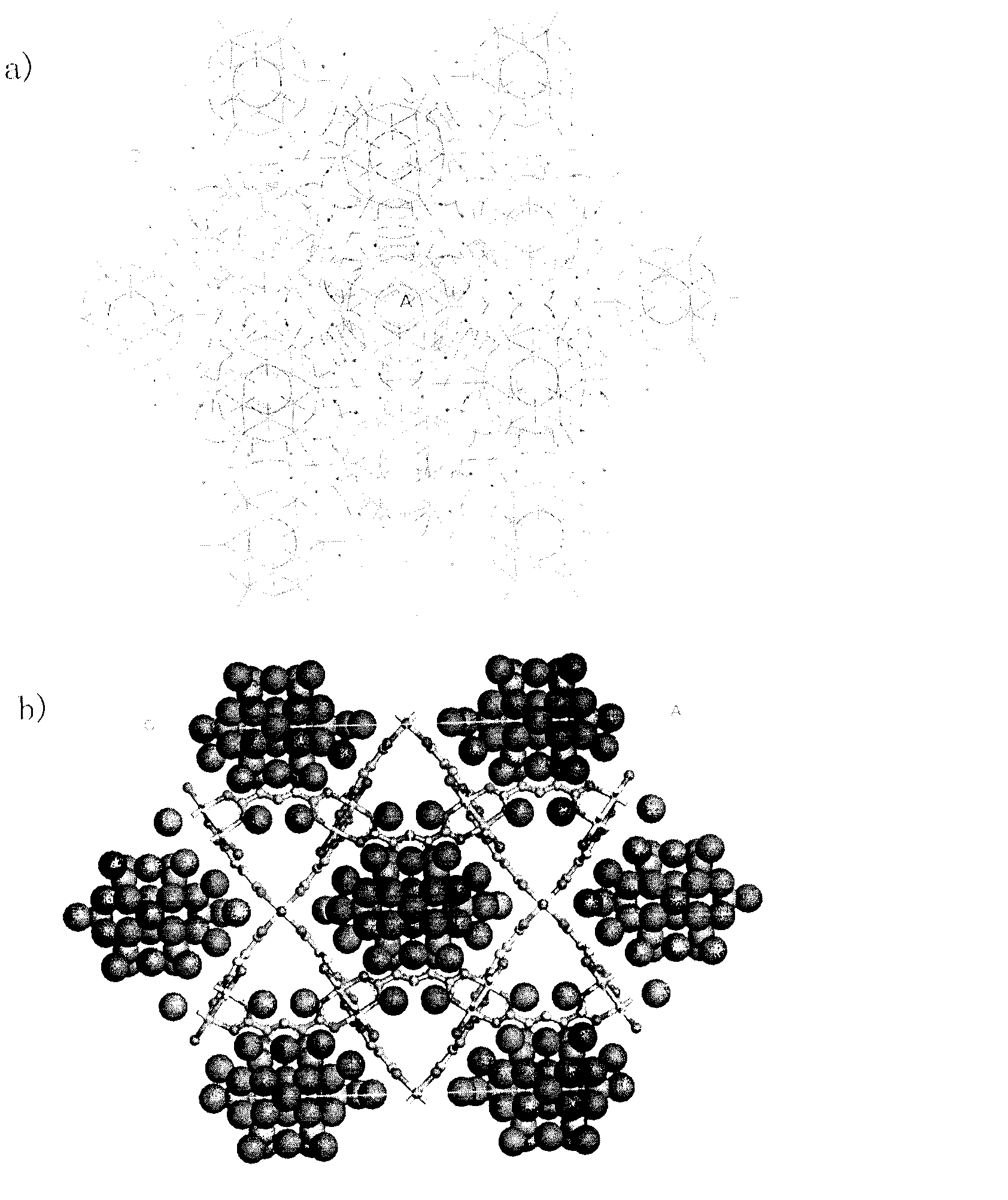
Copper system metal organic framework compound for selectively adsorbing heavy metal ions and preparation method thereof
A metal organic framework, heavy metal ion technology, applied in the directions of alkali metal compounds, alkali metal oxides/hydroxides, chemical instruments and methods, etc., can solve the problem of less research on adsorption performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] First weigh 0.8842g (3.66mmol) copper nitrate hexahydrate at room temperature, 0.8780g H 3 PW 12 o 40 (Cu / W=1) was dissolved in 12mL of deionized water, stirred at 1500r / min for 5min to obtain solution 1; at room temperature, weighed 0.4203g (2.00mmol) organic ligand trimesic acid and dissolved it in 12mL of absolute ethanol , stirred under 1500r / min rotating speed for 30min to obtain solution two; under room temperature, solution one and solution two were mixed evenly, and the mixed solution was poured into a quartz microwave reaction tube with a capacity of 35mL, and a microwave synthesizer (Discover series, U.S. CEM company , microwave maximum power 300W) Reaction parameters: reaction temperature 120°C, reaction time 20min, maximum withstand pressure 150psi, blue turbid liquid is obtained after microwave reaction; filter the blue turbid liquid at room temperature, and then use deionized water Wash with absolute ethanol five times to remove unreacted ligands and gue...
Embodiment 2
[0021] First weigh 0.8842g (3.66mmol) copper nitrate hexahydrate at room temperature, 0.8780g H 3 PW 12 o 40 (Cu / W=1) was dissolved in 12mL of deionized water, stirred at 1500r / min for 5min to obtain solution 1; at room temperature, weighed 0.4203g (2.00mmol) organic ligand trimesic acid and dissolved it in 12mL of absolute ethanol , stirred under 1500r / min rotating speed for 30min to obtain solution two; under room temperature, solution one and solution two were mixed evenly, and the mixed solution was poured into a quartz microwave reaction tube with a capacity of 35mL, and a microwave synthesizer (Discover series, U.S. CEM company , microwave maximum power 300W) Reaction parameters: reaction temperature 120°C, reaction time 20min, maximum withstand pressure 150psi, blue turbid liquid is obtained after microwave reaction; filter the blue turbid liquid at room temperature, and then use deionized water Wash with absolute ethanol five times to remove unreacted ligands and gue...
Embodiment 3
[0023] First weigh 0.8842g (3.66mmol) copper nitrate hexahydrate at room temperature, 0.8780g H 3 PW 12 o 40 (Cu / W=1) was dissolved in 12mL of deionized water, stirred at 1500r / min for 5min to obtain solution 1; at room temperature, weighed 0.4203g (2.00mmol) organic ligand trimesic acid and dissolved it in 12mL of absolute ethanol , stirred under 1500r / min rotating speed for 30min to obtain solution two; under room temperature, solution one and solution two were mixed evenly, and the mixed solution was poured into a quartz microwave reaction tube with a capacity of 35mL, and a microwave synthesizer (Discover series, U.S. CEM company , microwave maximum power 300W) Reaction parameters: reaction temperature 120°C, reaction time 20min, maximum withstand pressure 150psi, blue turbid liquid is obtained after microwave reaction; filter the blue turbid liquid at room temperature, and then use deionized water Wash with absolute ethanol five times to remove unreacted ligands and gue...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com