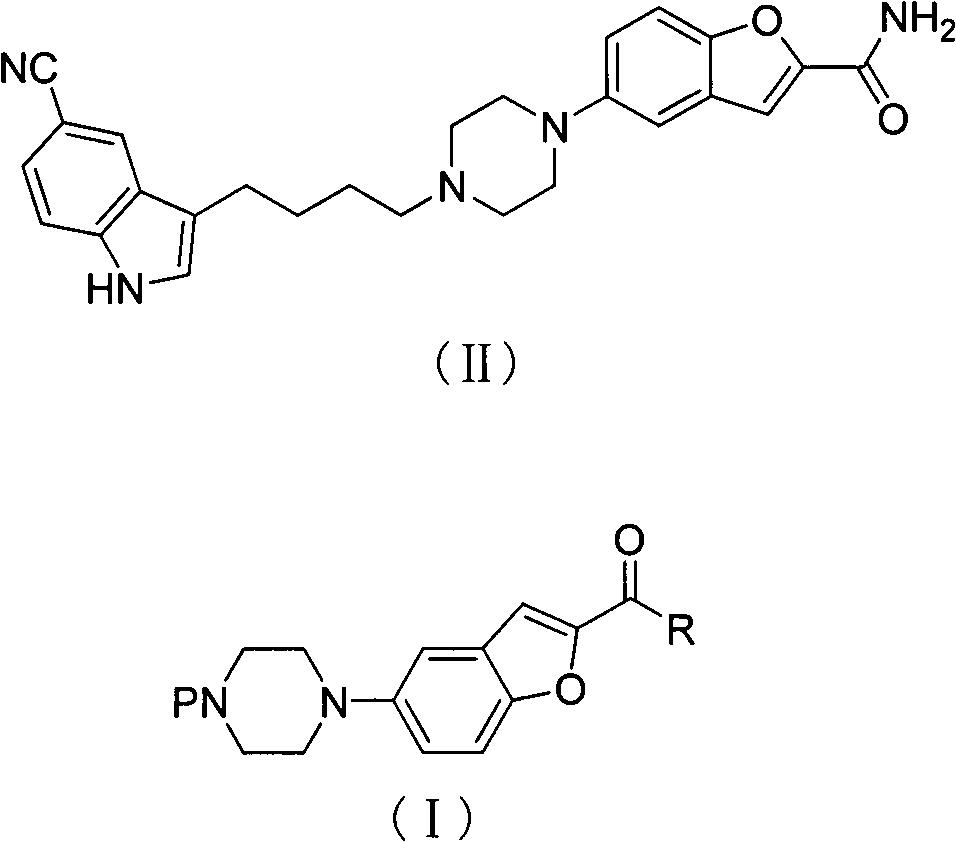
Method of preparing vilazodone intermediate 5-piperazin-2-acyl substituted benzofuran
A technology of acyl-substituted benzofuran, which is applied in the field of preparation of vilazodone intermediate 5-piperazinyl-2-acyl-substituted benzofuran, can solve the problems of cumbersome preparation, high price, and seriousness, and achieve improvement The effect of product yield, simplification of reaction steps, and little environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
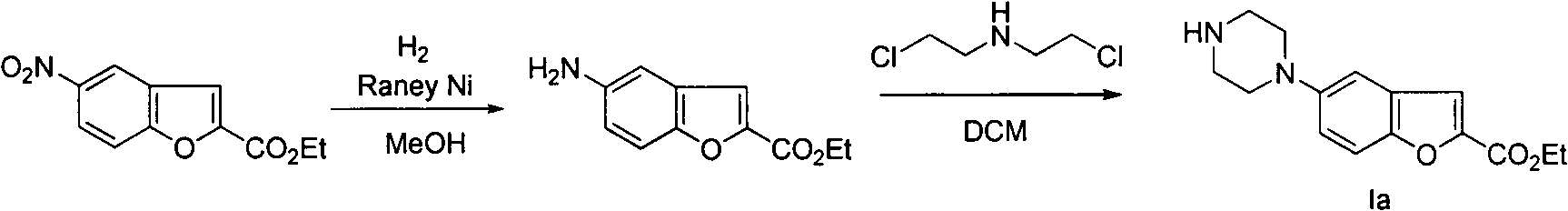
[0023] Example 1 Synthesis of 5-(4-N-tert-butoxycarbonylpiperazinyl)-2-carboxylic acid ethyl ester substituted benzofuran (Ia)
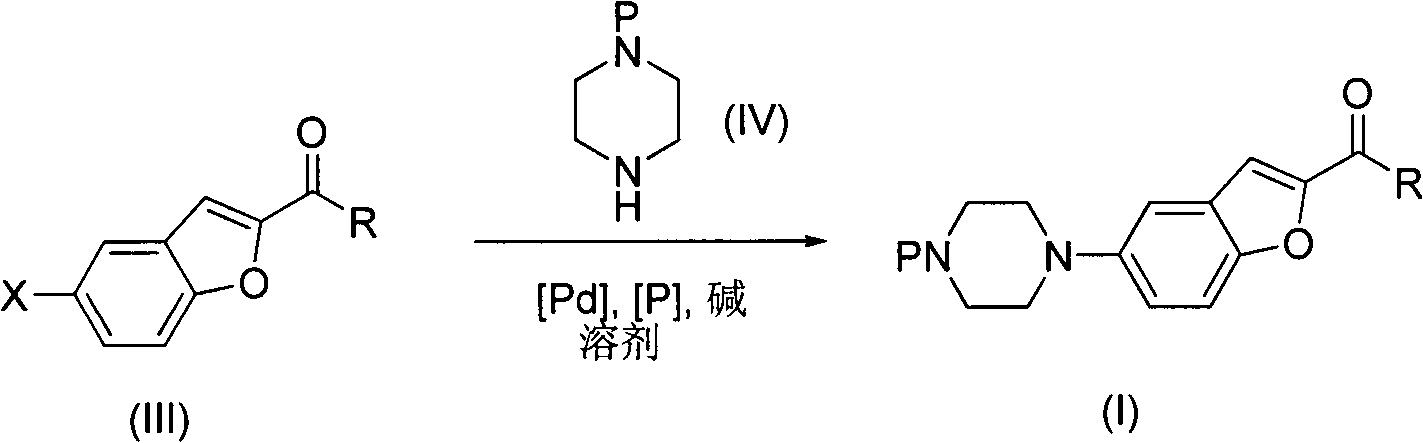
[0024] 27g 5-bromo-2-carboxylic acid ethyl ester substituted benzofuran (IIIa), 20g N-mono-tert-butoxycarbonyl substituted piperazine, 11.5g triphenylphosphine palladium, 5.2g triphenylphosphine and 22g tert-butyl Potassium alkoxide was added into 500 mL of toluene, and reacted at 100° C. for 24 hours. The solvent was removed under reduced pressure, 1 L of ether was added to the system, and a solid precipitated out. This solid was recrystallized from methanol to obtain 20.5 g of ethyl 5-(4-N-tert-butoxycarbonylpiperazinyl)-2-carboxylate substituted benzofuran (Ia).
Embodiment 2
[0025] Example 2 Synthesis of 5-(4-N-tert-butoxycarbonylpiperazinyl)-2-amide substituted benzofuran (Ib)
[0026] 24g 5-bromo-2-amide substituted benzofuran (IIIb), 20g N-mono-tert-butoxycarbonyl substituted piperazine, 2.5g palladium acetate, 12.5g 2,2'-bisdiphenylphosphino-1,1 '-Binaphthyl and 65g of cesium carbonate were added to 500mL of dimethylformamide and reacted at 100°C for 24 hours. The solvent was removed under reduced pressure, 1 L of ether was added to the system, and a solid precipitated out. This solid was recrystallized from methanol to obtain 24 g of 5-(4-N-tert-butoxycarbonylpiperazinyl)-2-amide substituted benzofuran (Ib).
Embodiment 3
[0027] Synthesis of Example Three 5-piperazinyl-2-carboxylic acid ethyl ester substituted benzofuran (Ic)
[0028] 27g 5-bromo-2-carboxylic acid ethyl ester substituted benzofuran (IIIa), 21g piperazine, 11.5g triphenylphosphine palladium, 5.2g triphenylphosphine and 65g cesium carbonate were added to 500mL toluene, at 100 °C for 24 hours. The solvent was removed under reduced pressure, 1 L of ether was added to the system, and a solid precipitated out. This solid was recrystallized from methanol to obtain 7.8 g of 5-piperazine-substituted-2-carboxylic acid ethyl ester-substituted benzofuran (Ic).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com