Ornidazole oral preparation and preparation method thereof
An oral preparation, ornidazole technology, applied in the field of pharmaceutical preparations, can solve problems such as complex prescriptions, and achieve the effects of simple prescriptions, controllable process, and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
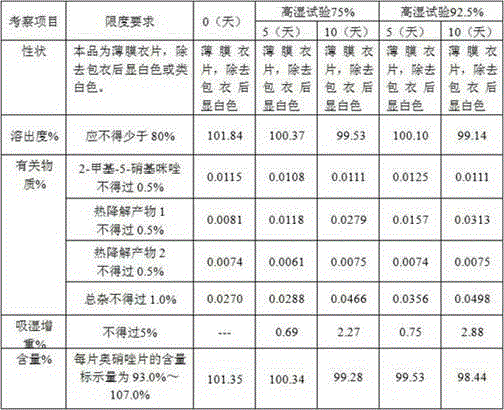
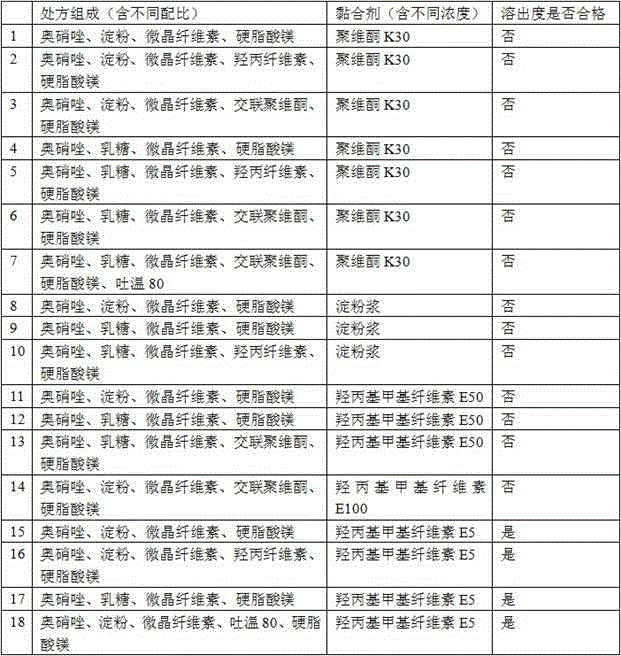
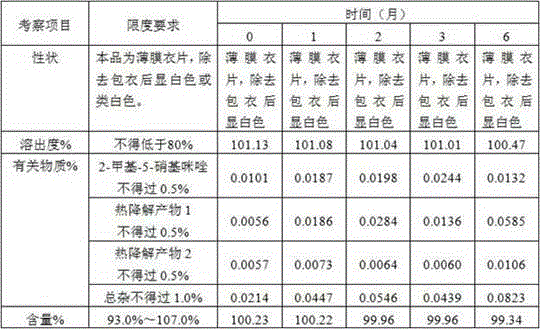
[0086] Prescription composition:
[0087] Ornidazole 5.0kg starch 0.4kg microcrystalline cellulose 0.8kg Adhesive 5% ( w / w ) Hydroxypropylmethylcellulose E5 aqueous solution Magnesium stearate 0.03kg production 10000 pieces
[0088] Preparation:
[0089] 1) Mix 5.0kg of ornidazole, 0.4kg of starch, and 0.8kg of microcrystalline cellulose, and add 5% of binder ( w / w ) 1.2-1.3 kg of hydroxypropyl methylcellulose E5 aqueous solution to prepare soft materials;
[0090] 2) Granulate with a 16-mesh screen, dry at 50-60°C for 10-30 minutes, use a stainless steel 16-mesh screen, granulate with a swinging granulator, and control the moisture content to ≤2%;
[0091] 3) Add lubricant magnesium stearate 0.5% and mix uniformly in a three-dimensional mixer;
[0092] 4) Detection content of intermediates;
[0093] 5) According to the results of the intermediate, calculate the weight of the tablet, and press the tablet to obtain the orn...
Embodiment 2
[0095] Prescription composition:
[0096] Ornidazole 5.0kg starch 0.3kg microcrystalline cellulose 0.9kg Adhesive 5% ( w / w ) Hydroxypropylmethylcellulose E5 aqueous solution Magnesium stearate 0.03kg production 10000 pieces
[0097] Preparation:
[0098] 1) Mix 5.0kg of ornidazole, 0.3kg of starch, and 0.9kg of microcrystalline cellulose evenly, and add 5% of binder ( w / w ) 1.2-1.3 kg of hydroxypropyl methylcellulose E5 aqueous solution to prepare soft materials;
[0099] 2) Granulate with a 16-mesh screen, dry at 50-60°C for 10-30 minutes, use a stainless steel 16-mesh screen, granulate with a swinging granulator, and control the moisture content to ≤2%;
[0100] 3) Add lubricant magnesium stearate 0.5% and mix uniformly in a three-dimensional mixer;
[0101] 4) Detection content of intermediates;
[0102] 5) According to the result of the intermediate, calculate the loading weight, put it into a hard gelatin capsule she...
Embodiment 3
[0104] Prescription composition:
[0105] Ornidazole 5.0kg starch 0.4kg microcrystalline cellulose 0.6kg Adhesive 5% ( w / w ) Hydroxypropylmethylcellulose E5 aqueous solution Magnesium stearate 0.03kg production 10000 pieces
[0106] Preparation:
[0107] 1) Mix 5.0kg of ornidazole, 0.4kg of starch, and 0.6kg of microcrystalline cellulose evenly, and add 1.2-1.3kg of 5% hydroxypropylmethylcellulose E5 aqueous solution of the binder to prepare a soft material;
[0108] 2) Granulate with a 16-mesh screen, dry at 50-60°C for 10-30 minutes, use a stainless steel 16-mesh screen, granulate with a swinging granulator, and control the moisture content to ≤2%;
[0109] 3) Add lubricant magnesium stearate 0.5% and mix uniformly in a three-dimensional mixer;
[0110] 4) Detection content of intermediates;
[0111] 5) According to the result of the intermediate, calculate the loading amount and put it into an aluminum-plastic bag to ob...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com