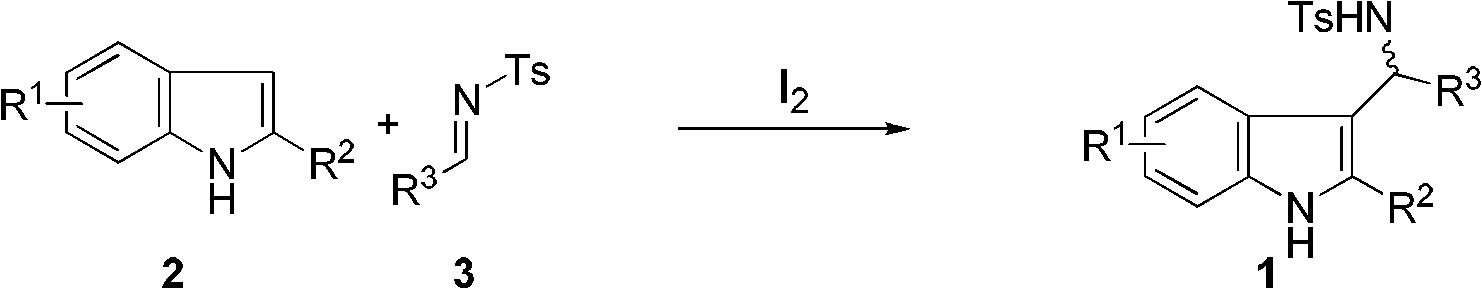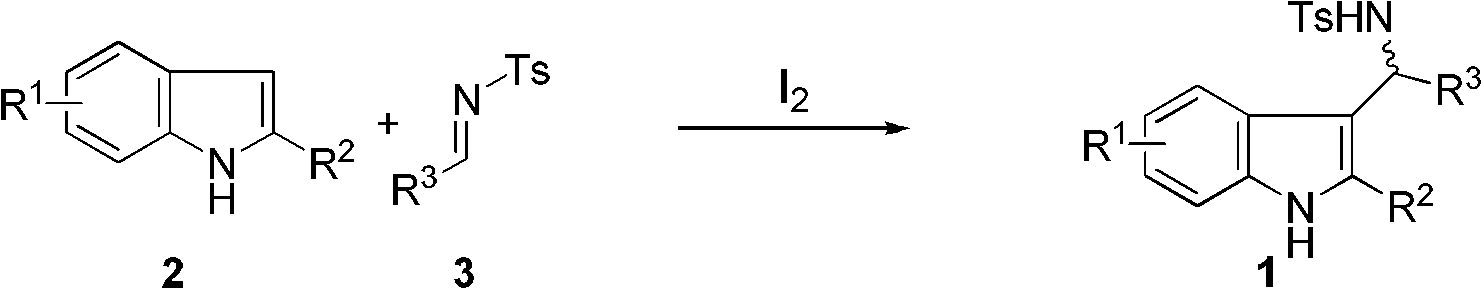Method for synthesizing 3-substituted indole and 2,3-disubstituted indole
A technology of indole and C7-C12, applied in the field of alkaloids, can solve the problems of low yield and achieve the effects of simple raw materials, shortened reaction time and high reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1: the synthesis of compound (1a)
[0028]
[0029] In a nitrogen-protected reaction flask, 2-methylindole (1.0 mmol) was added, followed by 4 ml of dry dichloromethane. After cooling to 0°C, elemental iodine (0.1 mmol) was added. After stirring and dissolving, imine (1.0 mmol) was added to the system. After constant temperature reaction for 5 min, saturated sodium thiosulfate solution was added to quench the reaction. After static separation, the aqueous layer was extracted three times with dichloromethane (3×3 mL). After combining the organic layers, they were dried over anhydrous sodium sulfate. The solvent was removed under reduced pressure, and the product compound 1 was obtained by silica gel column chromatography.
[0030] 4-Methyl-N-((2-methyl-1H-indol-3-yl)(phenyl)methyl)benzenesulfonamide (1a). Yield: 90%, white solid, 1 H NMR (400MHz, CDCl 3 )δ7.70(s, 1H), 7.46(d, J=8.0Hz, 2H), 7.39(d, J=7.4Hz, 2H), 7.29-7.11(m, 4H), 7.03(m, 4H), 6.88(t, J...
Embodiment 2
[0031] Embodiment 2: the synthesis of compound (1b)
[0032]
[0033] In a nitrogen-protected reaction flask, 2,7-dimethylindole (1.0 mmol) was added, followed by 4 ml of dry dichloromethane. After cooling to 0°C, elemental iodine (0.1 mmol) was added. After stirring and dissolving, imine (1.0 mmol) was added to the system. After constant temperature reaction for 5 min, saturated sodium thiosulfate solution was added to quench the reaction. The layers were separated, and the aqueous layer was extracted three times (3×3 mL). After combining the organic layers, they were dried over anhydrous sodium sulfate. The solvent was removed under reduced pressure, and the product compound was obtained by silica gel column chromatography.
[0034] N-((2,7-Dimethyl-1H-indol-3-yl)(phenyl)methyl)-4-methylbenzonesulfonamide (1g). Yield 99%, white solid, melting point: 144-145°C; 1 H NMR (400MHz, d 6 -Acetone) δ9.74(s, 1H), 7.50(d, J=7.9Hz, 2H), 7.42(d, J=7.6Hz, 2H), 7.24(t, J=7.4Hz, 2H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com