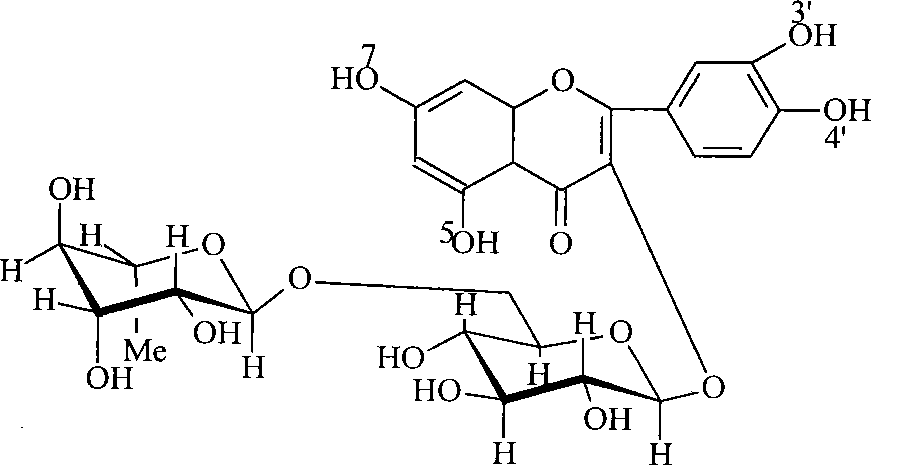
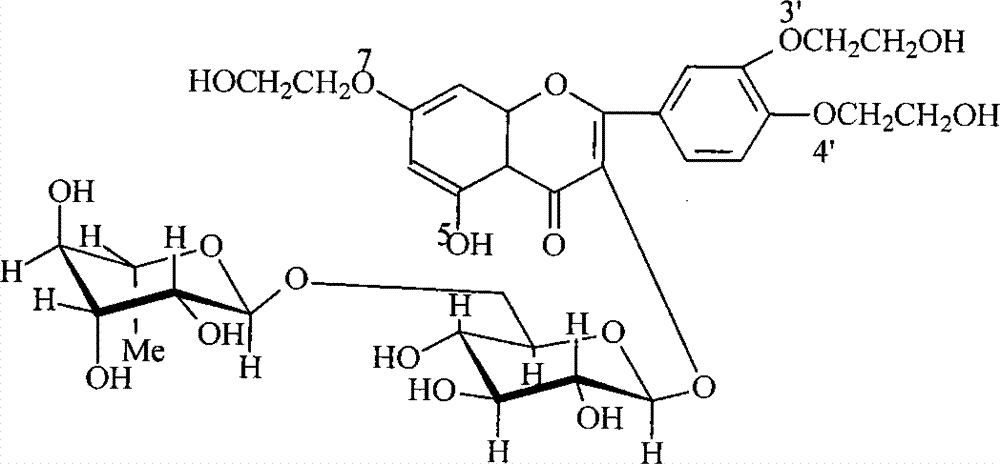
Preparation method of troxerutin
A rutin and reaction technology, applied in the field of chemical pharmacy, can solve the problems such as the difficulty of product purity, the inability to use 80-90°C, the difficulty of precise reaction conditions in industrial production control, and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] 100.0g of rutin dispersed in 300ml of water, add 1.0g of sodium hydroxide, after stirring evenly, add 220g of ethylene oxide, and react at 75°C for 3 hours. The reaction solution was taken to analyze the components, and the monohydroxyethylated product was 4%, the tetrahydroxyethylated product was 3%, and the di- and trihydroxyethylated products accounted for 93%. Add 62 g of borax, measure the pH of the reaction solution to 9.5, add 16 g of ethylene oxide, and continue to react at 70° C. for 3 hours. After the reaction was completed, the components of the reaction solution were analyzed: 88% of troxerutin, 7% of tetrahydroxyethyl product, and 3% of dihydroxyethyl product. Adjust the pH of the reaction solution to 6-7 with 6mol / L hydrochloric acid, concentrate to dryness under reduced pressure, add 400mL of methanol, then continue to adjust the pH to about 3 with dilute hydrochloric acid, heat to reflux, filter to remove insoluble matter, let it stand at room temperatur...
Embodiment 2
[0015] Dissolve 0.6g of sodium hydroxide in 150ml of water, add 53g of rutin (about 6% water content), and stir evenly. Add 12g of ethylene oxide into the reactor and react at 73°C for 3.5 hours. The reaction solution was taken for analysis, 3% of the monohydroxyethyl product, 5% of the tetrahydroxyethyl product, and 92% of the di- and tri-hydroxyethyl products. 31 g of borax was added, the pH of the reaction solution was 9.4, 4 g of ethylene oxide was added, and the reaction was continued at 70° C. for 2.5 hours. After finishing the reaction, the components of the reactant solution were analyzed: troxerutin accounted for 87%, tetrahydroxyethyl product was 9%, and dihydroxyethyl product was 2%. Use the calculated amount of concentrated hydrochloric acid to neutralize the borax to adjust the pH of the reaction solution to 5-7, concentrate and dry under reduced pressure, add 200mL of 90% ethanol, adjust the pH to about 3 with dilute hydrochloric acid, heat to about 75°C and sti...
Embodiment 3
[0017] Dissolve 0.7g of potassium hydroxide in a mixed solution of 150ml of water and 50ml of methanol, add 55g of rutin with a water content of about 6%, and stir evenly. Add 11 g of ethylene oxide into the reactor and react at 73° C. for 3 hours. Add 30 g of borax and continue to add 6 g of ethylene oxide, and continue to react at 70° C. for 3 hours. Finish reaction, analysis reactant troxerutin accounts for 90%, dihydroxyethyl product 3%, tetrahydroxyethyl product 6%. The aftertreatment is similar to Example 1, and the yield and purity are comparable.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com