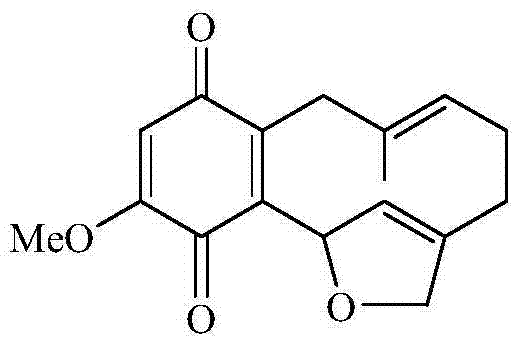
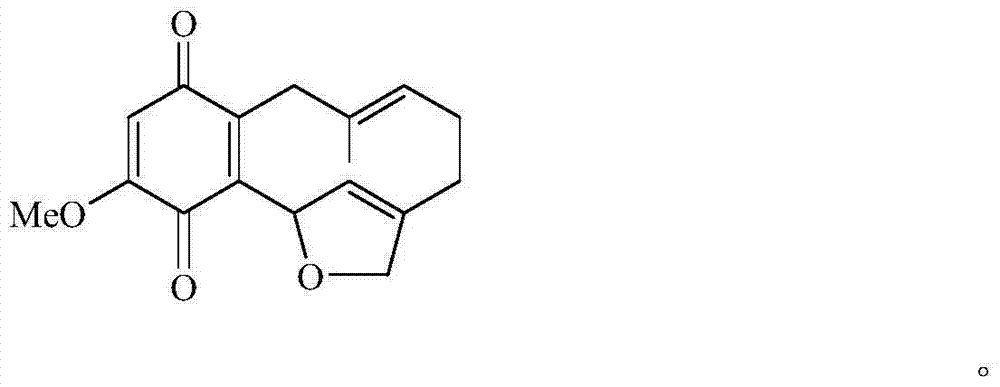
Benzoquinone compound and use thereof for preparing anti-tumour drug
An anti-tumor drug and compound technology, applied in anti-tumor drugs, drug combinations, organic chemistry, etc., can solve the problem of affecting tumor cell metabolism, proliferation and differentiation, hindering tumor cell growth, and no anti-tumor efficacy of benzoquinone compounds. Oncology drugs and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Embodiment 1 prepares shikonin
[0016] Comfrey root coarse powder 20kg, at room temperature with 95% ethanol after repeated cold soaking, percolation and extraction for several times, the solvent was recovered under reduced pressure to obtain 820g of extractum, which was suspended in distilled water and mixed with petroleum ether, ethyl acetate and n-butanol Extraction to obtain 220g of petroleum ether extract, 420g of ethyl acetate extract and 180g of n-butanol extract; 200g of ethyl acetate extract was mixed with silica gel and dried, followed by silica gel column chromatography, followed by petroleum ether, petroleum ether- Acetone and acetone were used for gradient elution to obtain 9 fractions Fr.1~Fr.9; the obtained fraction Fr.6 was subjected to petroleum ether-acetone (8:1,5:1,5:2,2:1) silica gel Column chromatography obtained 4 eluted fractions of Fr.6A~Fr.6D, among which Fr.6C was recrystallized from petroleum ether-acetone to obtain the compound shikonone B ...
Embodiment 2
[0017] Example 2 In vitro cytotoxicity test
[0018] Four kinds of tumor cell lines A549, DU145, KB and their drug-resistant strain KB-VIN were cultured in 10% calf serum RPMI1640 containing 25mM HEPES, 0.2% (w / v) sodium bicarbonate and 100μg / ml kanamycin 4ml T-25 flask, 37°C, 5% CO 2 cultivated under conditions. The cell suspension digested by trypsin was added to a 96-well plate, and the cell concentration was 0.25-1×10 4 / hole. Tumor cells were added different concentrations of test compound samples and cultured at 37°C for 72 hours, fixed with ice-cold 50% trichloroacetic acid and stained with 0.4% SRB. After the dye was dissolved, the absorbance was measured at 562nm. ED 50 The drug concentration at the time of half cell growth inhibition was converted according to the dose-effect data. Each experiment was repeated three times with less than 5% difference in absorbance values, ED 50 The difference is less than 30%. to ED 50 ≤20μg / ml is the effective standard. The ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com