Preparation of silk-like non-precious-metal nanotube oxygen reduction electrocatalyst
An electrocatalyst and nanotube technology, applied in the field of electrocatalysis, can solve the problems of limited practical application and complete commercialization, troubled by cost and life problems, lack of precious metal Pt resources, etc., achieving good methanol poisoning ability, low production cost, Catalytic activity and stability of aerobic reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
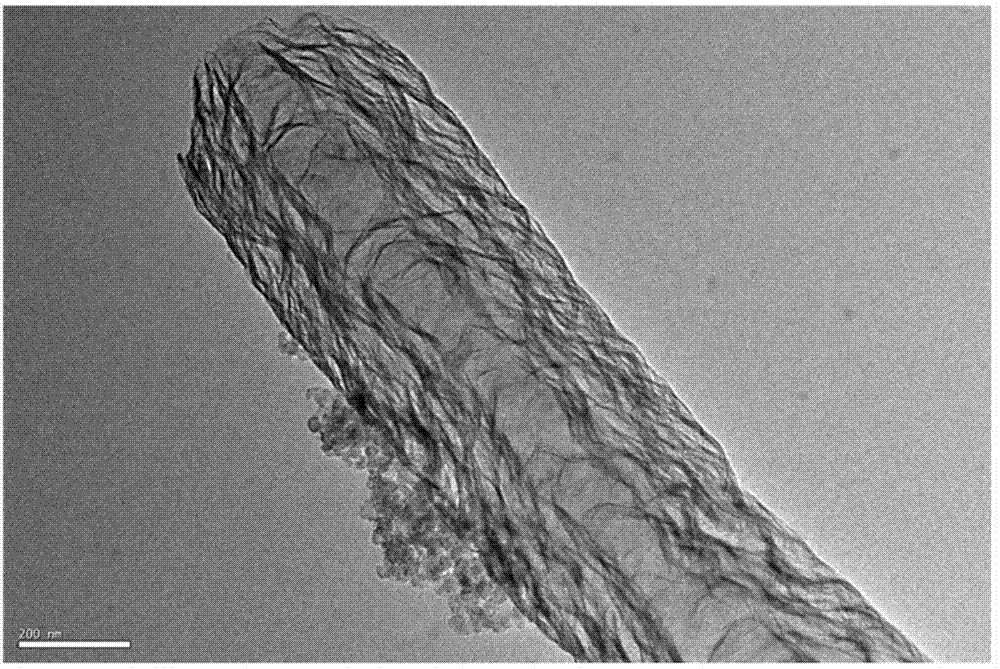
[0029] Dissolve 2.0 g of L-lysine in a flask filled with 30 mL of aqueous ethanol and sonicate until completely dissolved, and dissolve 2 mL of FeCl with a concentration of 0.05 g / mL 3 Add the solution dropwise into the above flask, stir and sonicate for 30 min, then add 0.4 g of silica nanospheres (d=30±10 nm) into the above flask, stir and sonicate for 60 min, then evaporate it on a rotary evaporator. To dry, simply dry in the oven. The obtained samples were heated to 300°C at a rate of 5°C / min and stayed for 1 h in a tube furnace under the protection of nitrogen, then raised to 900°C and taken out after staying for 1 h. The silica template was removed from the hydrofluoric acid solution, filtered with suction, washed with water, and dried to obtain the catalyst.
[0030] The half-wave potential of the catalyst for catalytic oxygen reduction in 0.1 M KOH saturated oxygen solution is -96.7 mV, which is 23.6 mV earlier than the commercial Pt / C (Jonhson-Matthey Company) cataly...
Embodiment 2
[0032] Dissolve 2.0 g of arginine in a flask containing 40 mL of ethanol aqueous solution and sonicate until it is completely dissolved, add 3 mL of NiCl solution with a concentration of 0.04 g / mL into the above flask, stir and sonicate for 50 min, and then dissolve 0.8 g The silica nanospheres were added into the above flask, stirred and ultrasonicated for 60 min, evaporated to dryness on a rotary evaporator, and dried in an oven. The obtained samples were heated to 400°C at a rate of 5°C / min in a tube furnace under the protection of nitrogen and stayed for 1 h, then raised to 900°C and taken out after staying for 1 h. The silica template is removed from the hydrofluoric acid solution, filtered with suction, washed with water and dried to obtain the catalyst.
[0033] The half-wave potential of the catalyst for catalytic oxygen reduction in 0.1 M KOH saturated oxygen solution is -100.7 mV, which is 19.6 mV earlier than the commercial Pt / C (Jonhson-Matthey Company) catalyst. ...
Embodiment 3
[0035] Dissolve 2.0 g of histidine in a flask containing 50 mL of ethanol aqueous solution and sonicate until completely dissolved, and add 4 mL of 0.04 g / mL CoCl 2The solution was added dropwise to the above flask, stirred and ultrasonicated for 60 min, then 0.8 g of silica nanospheres were added to the above flask, stirred and ultrasonically 60 min, evaporated to dryness on a rotary evaporator, and dried in an oven. The obtained samples were heated to 250°C at a rate of 5°C / min in a tube furnace under the protection of nitrogen, stayed for 1 h, then raised to 900°C, and taken out after staying for 1.5 h. The silica template was removed from the hydrofluoric acid solution, filtered with suction, washed with water, and dried to obtain the catalyst.
[0036] The half-wave potential of the catalyst for catalytic oxygen reduction in 0.1 M KOH saturated oxygen solution is -99.7 mV, which is 20.6 mV earlier than the commercial Pt / C (Jonhson-Matthey Company) catalyst.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com