Ribavirin small-capacity injection preparation and preparation method thereof
A ribavirin and small-capacity technology, applied in the field of medicine, can solve problems such as high loss and affecting content, and achieve high yield, reduce pain, and safe clinical use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] A small volume injection of ribavirin comprises the following components by weight percentage:
[0024] 500 sticks (2ml: 0.25g)
[0025] Ribavirin 12.5%,
[0026] Activated carbon 0.01%,
[0027] Benzyl Alcohol 4%,
[0028] Add water for injection to 100%;
[0029] Acetic acid-sodium acetate buffer Adjust the pH to 5.0.
[0030] The production method is as follows:
[0031] (1) Weigh 50% of the water for injection of the formula and cool it to about 40°C;
[0032] (2) Dissolve ribavirin and analgesic benzyl alcohol in the cooled water for injection in step (1) in the formulated amount;
[0033] (3) Weigh the amount of activated carbon in the formula and add it to the solution obtained in step (2), stir well and let it stand for 15 minutes, filter and decarbonize, and add water for injection to the full amount;
[0034] (4) Filter the solution obtained in step (3) with a titanium rod filter and circulate for 25 minutes;
[0035] (5) After filtering the solution o...
Embodiment 2
[0038] A small volume injection of ribavirin comprises the following components by weight percentage:
[0039] 1000 sticks (1ml: 0.1g)
[0040] Ribavirin 10%;
[0041] Activated carbon 0.015%;
[0042] Benzyl alcohol 2%;
[0043] Add water for injection to 100%;
[0044] Disodium hydrogen phosphate-sodium dihydrogen phosphate buffer to adjust the pH to 5.8.
[0045] The production method is as follows:
[0046] (1) Weigh 50% of the water for injection of the formula and cool it to about 40°C;
[0047] (2) Dissolve ribavirin and analgesic benzyl alcohol in the cooled water for injection in step (1) in the formulated amount;
[0048] (3) Weigh the amount of activated carbon in the formula and add it to the solution obtained in step (2), stir well and let it stand for 15 minutes, filter and decarbonize, and add water for injection to the full amount;
[0049] (4) Filter the solution obtained in step (3) with a titanium rod filter and circulate for 30 minutes;
[0050] (5)...
Embodiment 3
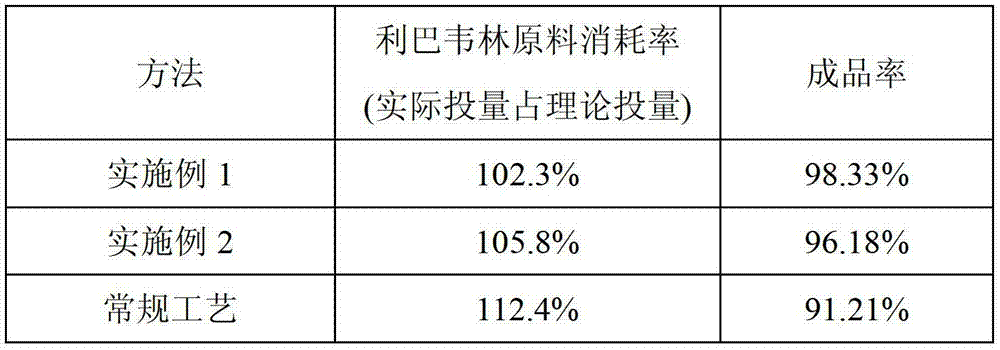
[0052] Embodiment 3: The inspection result of yield rate.
[0053] Product yield and raw material consumption rate of ribavirin small-capacity injection (according to 1L volume) prepared by Example 1, 2 and the current conventional process method (what process, please cite references, or briefly describe!) As shown in the following table:
[0054]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com