Prescription and preparation process for irinotecan hydrochloride injection
A technology of irinotecan hydrochloride and preparation process, which can be applied in directions such as pharmaceutical formulation, drug delivery, and drug combination, can solve problems such as lack of effective drugs, and achieve the effects of good sterilization effect, convenient use and easy production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Take precisely weighed lactic acid, add 20%-30% of the proportioned amount of water for injection at 75°C to 85°C, stir to dissolve, then slowly pour the precisely weighed irinotecan hydrochloride into the lactic acid solution and stir evenly, then quickly Add 60% to 70% of the dosed water for injection at 75°C to 85°C and fully stir until completely dissolved, then add precisely weighed sorbitol, and stir at a speed of 1200-1500rpm for more than 20 minutes until completely dissolved. Add 0.1% activated carbon (W / V), stir and adsorb at 900-1100rpm for 30 minutes. Decarbonize with a 0.45μm metal filter. Add water for injection to a dosage of 95% (mass percentage), adjust the pH value to 3.2-3.6 with a concentration of about 0.2 mol / L sodium hydroxide solution or hydrochloric acid, add water for injection to 30L, and stir at a speed of 900-1100 rpm for 5 ~15 minutes. 0.22μm microporous membrane filtration. Filling, stoppering and capping, sterilization (sterilization i...
Embodiment 2
[0031] Take precisely weighed lactic acid, add 20%-30% of the proportioned amount of water for injection at 75°C to 85°C, stir to dissolve, then slowly pour the precisely weighed irinotecan hydrochloride into the lactic acid solution and stir evenly, then quickly Add 60% to 70% of the dosed water for injection at 75°C to 85°C and fully stir until completely dissolved, then add precisely weighed sorbitol, and stir at a speed of 1200-1500rpm for more than 20 minutes until completely dissolved. Add 0.1% activated carbon (W / V), stir and adsorb at 900-1100rpm for 30 minutes. Decarbonize with a 0.45μm metal filter. Add water for injection to a dosage of 95% (mass percentage), adjust the pH value to 3.2-3.6 with a concentration of about 0.2 mol / L sodium hydroxide solution or hydrochloric acid, add water for injection to 30L, and stir at a speed of 900-1100 rpm for 5 ~15 minutes. 0.22μm microporous membrane filtration. Filling, stoppering and capping, sterilization (moist heat ster...
Embodiment 3
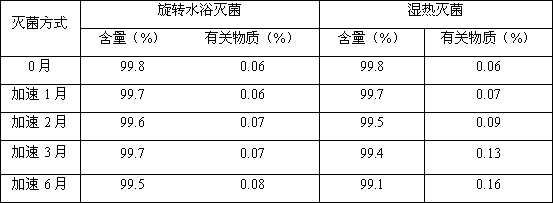
[0033] The accelerated stability study of irinotecan hydrochloride injection prepared by two sterilization methods is carried out, and the experimental results are compared as follows:
[0034]
[0035] Conclusion: From the above comparative experiments, the results show that the sterilization effect of rotating water is good, which can effectively control the increase of related substances in irinotecan hydrochloride injection, prolong the validity period, and make the drug quality and safety controllable.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com