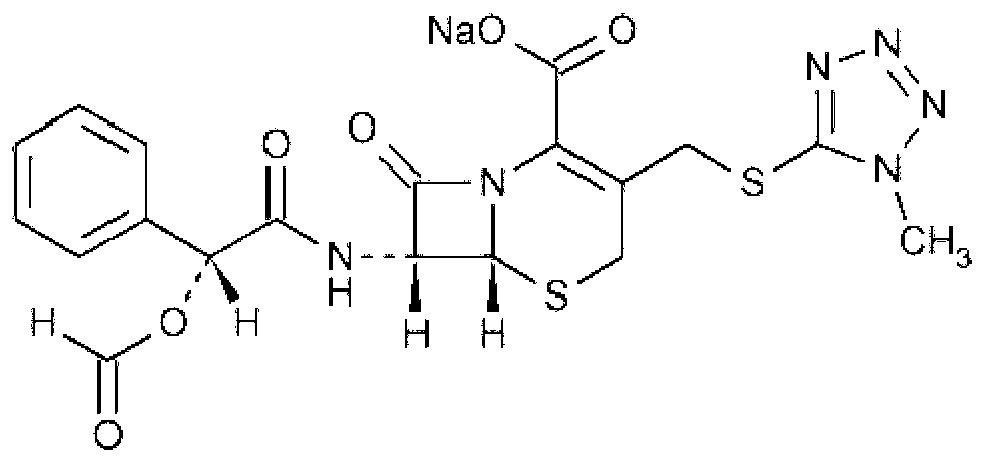
Method for refining cefamandole nafate, cefamandole nafate and application thereof
A technology of cefamandole sodium and its refining method, which is applied in the processing of cefamandole sodium, the refining of cefamandole sodium, the application in the preparation of antibacterial drugs, and the field of sterile composition powder injection, which can solve the problem of stability Poor, poor solubility, deep color and other problems, to achieve the effect of reducing the generation of degradation products and other impurities, good solubility, and good clarity of the solution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] This embodiment is used to illustrate the refining method of cefamandole sodium of the present invention.
[0035] (1) Prepare 3L of mixed solvent with methanol and ethyl acetate at a volume ratio of 1:5, stir evenly, and set aside.
[0036] (2) Take 500 g of crude cefamandole sodium at room temperature, dissolve it in 2 L of water for injection, stir until all the solids are dissolved, add dropwise 10% aqueous sodium acetate to adjust the pH to 6.5, and obtain a crude solution.
[0037] (3) Add the above mixed solvent into the crude product solution, stir while adding, keep the reaction temperature at 20-25°C during the dropping process, and the dropping rate is 0.25L / min, and a white solid is found to precipitate from the solution. After the dropwise addition, continue to add 1L of ethyl acetate dropwise to obtain a suspension.
[0038] (4) Cool the obtained suspension to 3-5°C, let the crystal grow for 24 hours, filter under reduced pressure, and dry in vacuum at 40...
Embodiment 2
[0041] This embodiment is used to illustrate the refining method of cefamandole sodium of the present invention.
[0042] (1) Prepare 3L of mixed solvent with methanol and ethyl acetate at a volume ratio of 1:10, stir evenly, and set aside.
[0043] (2) Take 500 g of crude cefamandole sodium at room temperature, dissolve it in 2 L of water for injection, stir until all the solids are dissolved, add dropwise 10% aqueous sodium acetate to adjust the pH to 6.5, and obtain a crude solution.
[0044] (3) Add the above mixed solvent into the crude product solution, stir while adding, keep the reaction temperature at 20-25°C during the dropping process, and the dropping rate is 0.25L / min, and a white solid is found to precipitate from the solution. After the dropwise addition, continue to add 1L of ethyl acetate dropwise to obtain a suspension.
[0045] (4) Cool the resulting suspension to 3-5°C, let it grow for 24 hours, filter under reduced pressure, and dry it in vacuum at 40°C t...
Embodiment 3
[0048] This embodiment is used to illustrate the refining method of cefamandole sodium of the present invention.
[0049] (1) Prepare 3L of mixed solvent with ethanol and ethyl acetate at a volume ratio of 1:5, stir well, and set aside.
[0050] (2) Take 500 g of crude cefamandole sodium at room temperature, dissolve it in 2 L of water for injection, stir until all the solids are dissolved, add dropwise 10% aqueous sodium acetate to adjust the pH to 6.5, and obtain a crude solution.
[0051] (3) Add the above mixed solvent into the crude product solution, stir while adding, keep the reaction temperature at 20-25°C during the dropping process, and the dropping rate is 0.25L / min, and a white solid is found to precipitate from the solution. After the dropwise addition, continue to add 1L of ethyl acetate dropwise to obtain a suspension.
[0052] (4) Cool the resulting suspension to 3-5°C, let it grow for 24 hours, filter under reduced pressure, and dry it in vacuum at 40°C to ob...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com