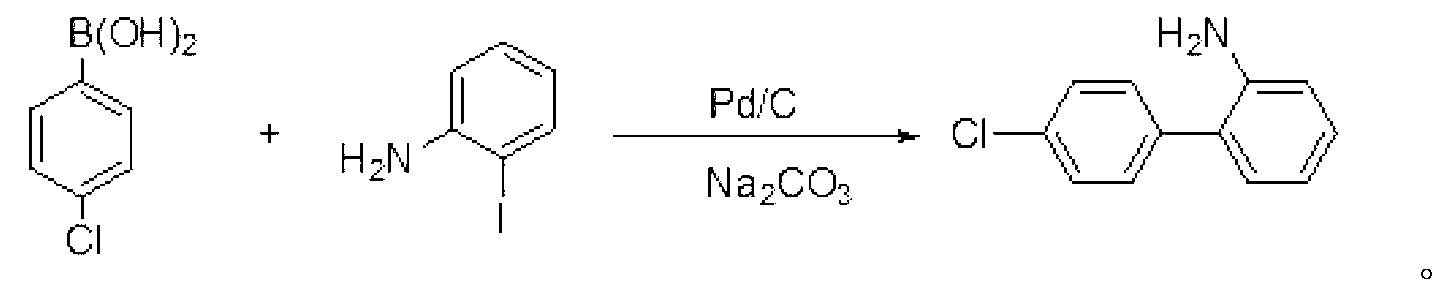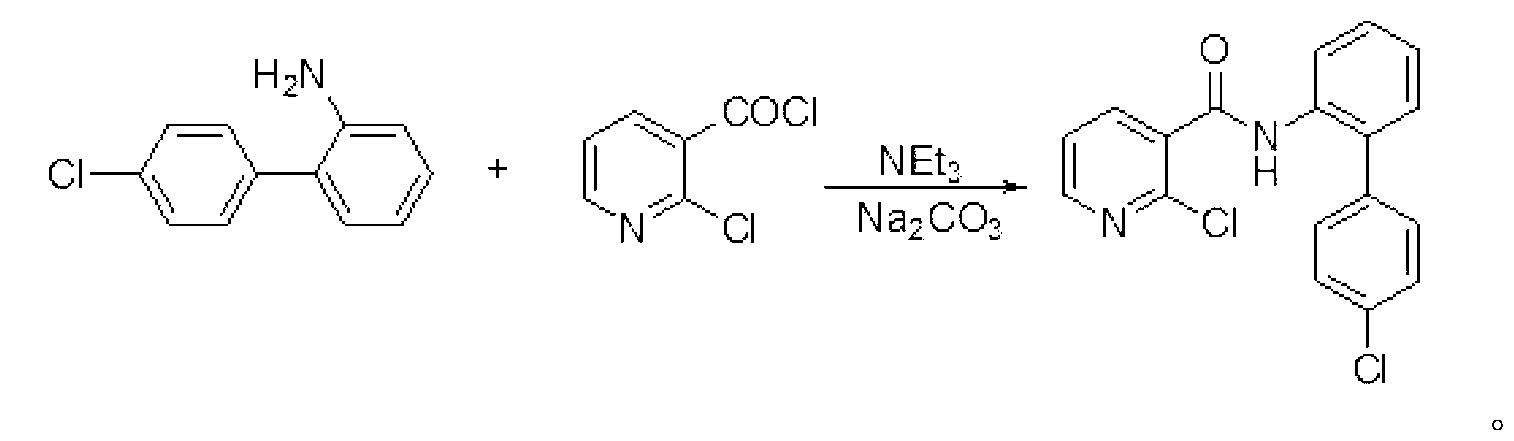Preparation method of Boscalid
A technology of boscalid and iodoaniline, applied in the field of fungicide preparation, can solve the problems of low yield, high cost, harsh reaction conditions, etc., and achieve the effect of reducing production cost and increasing yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Embodiment 1: the preparation of 4'-chlorobiphenyl-2-amine
[0018] Add 4-chlorophenylboronic acid (31.3g, 200mmol), o-iodoaniline (39.2g, 180mmol), Pd / C (0.3g, 5%) and toluene (300mL) into a 1L three-necked flask, and stir at room temperature for 15 minutes. Then add Na 2 CO 3 Aqueous solution (300mL, 25%), the mixture was heated and stirred to reflux for 30h, then cooled to room temperature, and the layers were separated. The inorganic layer was extracted twice with toluene (50 mL×2), and the organic layers were combined and washed three times with water (100 mL×3). Anhydrous MgSO for organic phase 4 Dry and recover toluene under reduced pressure. The remaining oil was put into the refrigerator at 0~5°C to freeze to obtain 34.2 g of brown solid, yield: 93.2%. Mp: 39-41°C; (literature value CAS: 1204-44-0, mp: 42-44°C).
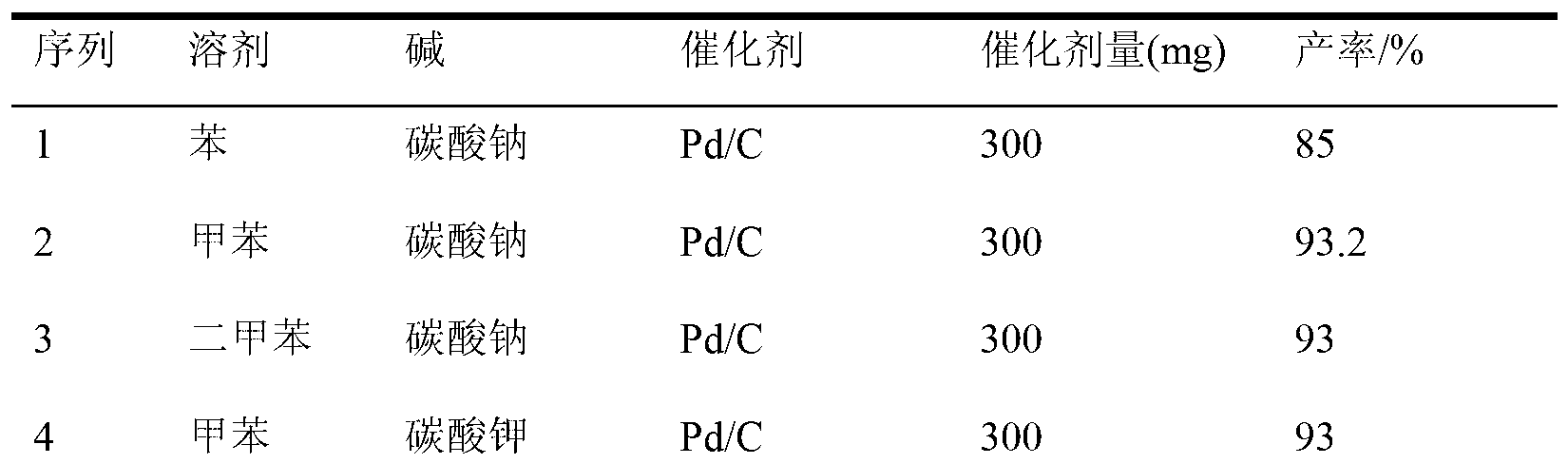
[0019] According to the above method, using o-iodoaniline and 4-chlorophenylboronic acid as raw materials, react under different solvents, diff...
Embodiment 2
[0024] Embodiment 2: the preparation of boscalid
[0025] In a dry 250 mL flask add 4'-chlorobiphenyl-2-amine (20.4 g, 100 mmol), dry dichloroethane (150 mL), sodium carbonate (21.2 g, 200 mmol), and a catalytic amount of 1 mL of triethylamine . Then it was cooled to 10°C with ice water, and 2-chloronicotinoyl chloride (19.36 g, 110 mmol) was added in 5 batches (about 4 g per batch), and then the reaction was continued at room temperature for 4 hours. After the reaction, wash with water (30mL), then with hydrochloric acid (20mL, 10%), and finally with water (30mL). Anhydrous MgSO for organic phase 4 After drying, the solvent was recovered by atmospheric distillation, and the remaining solid was recrystallized with a mixed solvent of alcohol and water (volume ratio: 1:1) to obtain 31.2 g of boscalid, with a yield of 91.1%. Mp: 143-144°C; (literature value CAS: 188425-85-6, mp: 142-144°C).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com