Method for detecting protease activity and inhibitor activity thereof based on chemiluminescence
A protease inhibitor, chemiluminescence technology, used in chemiluminescence/bioluminescence, analysis by chemical reaction of materials, etc., can solve the problems of cumbersome operation, high cost, low detection sensitivity, etc., to protect the environment, reduce Pollution, simple operation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0094] Example 1 Detection of trypsin activity
[0095] Step 1. Take nine 3 mL sample tubes and add cytochrome C with a final concentration of 50 nM respectively. Cytochrome C is mixed with Tris-HCl buffer solution (1 mM CaCl 2 ) diluted, pre-incubated in a 37°C water bath for 5 minutes;
[0096]Step 2: Add trypsin solutions with final concentrations of 0, 2, 5, 10, 20, 50, 100, 150, and 200 ng / mL to the 9 sample tubes in step 1, and mix them evenly immediately. The volume is 3mL to obtain a hydrolyzed solution;
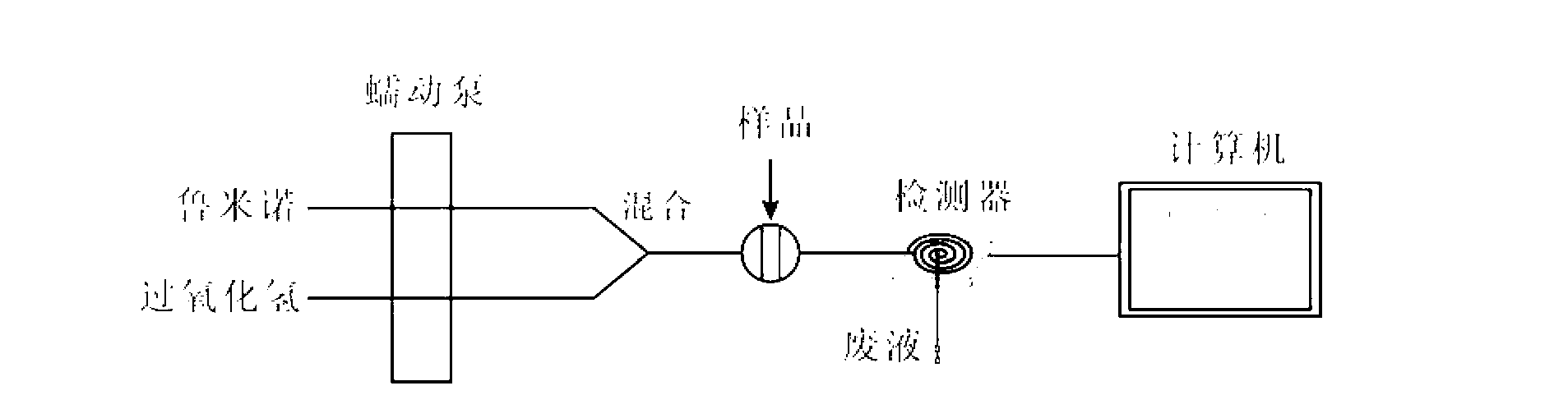
[0097] Step 3: Take 200 μL of the hydrolysis solution in Step 2 and inject it into the flow injection chemiluminescence flow path, and undergo a chemiluminescent reaction with the luminol-hydrogen peroxide detection system. As the sample and the detection system flow through the detector, the generated chemical The luminescence is detected by the detector, collected and displayed on the display by the data acquisition system, and the chemiluminescence intensity val...
Embodiment 2
[0099] Example 2 Detection of proteinase K activity
[0100] Step 1. Take nine 3 mL sample tubes and add cytochrome C with a final concentration of 50 nM respectively. Cytochrome C is mixed with Tris-HCl buffer solution (1 mM CaCl 2 ) diluted, pre-incubated in a 35°C water bath for 10 minutes;
[0101] Step 2: Add proteinase K solution with a final concentration of 0, 2, 5, 10, 20, 50, 100, 150, and 200 ng / mL to the 9 sample tubes in step 1 in sequence, and mix them evenly immediately. The volume is 3mL to obtain a hydrolyzed solution;
[0102] Step 3: Take 180 μL of the hydrolysis solution in Step 2 and inject it into the flow injection chemiluminescence flow path, and undergo a chemiluminescence reaction with the luminol-hydrogen peroxide detection system. As the sample and the detection system flow through the detector, the generated chemical The luminescence is detected by the detector, collected and displayed on the display by the data acquisition system, the chemilumin...
Embodiment 3
[0104] Example 3 The detection of papain activity
[0105] Step 1. Take eight 3 mL sample tubes and add cytochrome C with a final concentration of 50 nM respectively. Cytochrome C is mixed with 50 mM Tris-HCl buffer solution (1mM CaCl 2 ) diluted, pre-incubated in a water bath at 40°C for 3 minutes;
[0106] Step 2: Add the papain solution with a final concentration of 0, 5, 10, 20, 50, 100, 200, 1000 ng / mL to the 8 sample tubes in step 1, mix immediately, and the total volume of the sample is 3mL to obtain a hydrolyzed solution;
[0107] Step 3: Inject 150 μL of the hydrolyzed solution in Step 2 into the flow injection chemiluminescent flow path, and undergo a chemiluminescent reaction with the luminol-hydrogen peroxide detection system. As the sample and the detection system flow through the detector, the generated chemical The luminescence is detected by the detector and collected and displayed on the display by the data acquisition system. The chemiluminescence intensity...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com