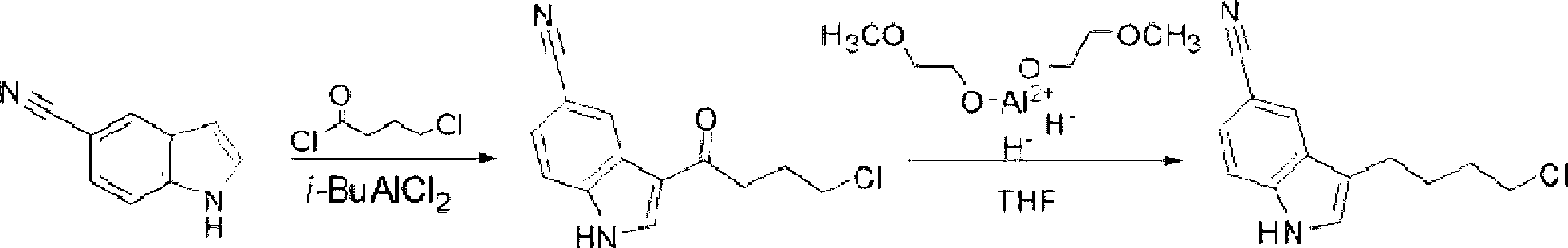
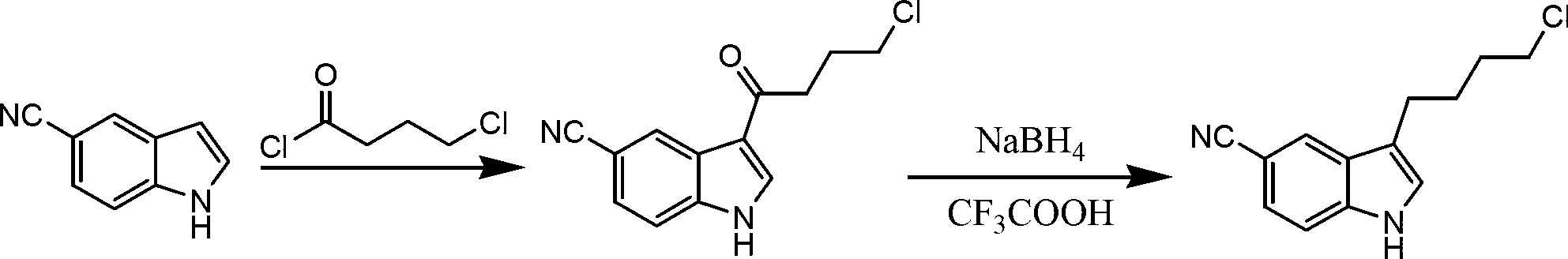
Preparation method of 3-(4-chlorobutyl)indole-5-formonitrile
A technology of chlorobutyl and chlorobutyryl, which is applied in the field of preparation of 3-indole-5-carbonitrile, can solve the problems of difficult industrial production, expensive reagents, cumbersome operation, etc., and achieve low production cost and large implementation Value and socio-economic benefits, effects on increased yield and product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Add 50g of 5-cyanindole, 500ml of dichloromethane, and 49.6g of 4-chlorobutyryl chloride into a 1L reaction bottle, cool down to -5°C in an ice bath, add 46.9g of aluminum trichloride in four times, and keep the temperature at -5°C. Stir and react at -0°C for 5 hours, and treat the reaction liquid by conventional methods to obtain 78.1 g of 3-(4-chlorobutyryl)indole-5-carbonitrile; add 800 ml of dichloromethane to the obtained intermediate, and cool down to -5°C in an ice bath , add 24g of sodium borohydride in four times, add 72.2g of trifluoroacetic acid dropwise after addition, keep the temperature below 0°C for 5h, after the reaction is completed, follow the conventional method to obtain 3-(4-chlorobutyl)indole-5 -Formonitrile 61.4g, total yield 75%, HPLC purity 99.5%.
Embodiment 2
[0031] Add 50g of 5-cyanindole, 400ml of dichloromethane, 100ml of nitromethane, 99.2g of 4-chlorobutyryl chloride into a 1L reaction bottle, cool to -5°C in an ice bath, add 93.8g of aluminum trichloride in four times, Keep the temperature at -5-0°C, stir and react for 5 hours, and treat the reaction solution by conventional methods to obtain 75g of 3-(4-chlorobutyryl)indole-5-carbonitrile; add 346.6g of trifluoroacetic acid to the obtained intermediate, and keep the temperature Add 57.6g of sodium borohydride four times below 0°C, keep warm at -5-0°C and stir for 5 hours after the addition, after the reaction is completed, follow the conventional method to obtain 3-(4-chlorobutyl)indole-5-carbonitrile 63g, total yield 77%, HPLC purity 99.3%.
Embodiment 3
[0033] Add 50g of 5-cyanindole, 500ml of petroleum ether, and 74.4g of 4-chlorobutyryl chloride into a 1L reaction bottle, cool down to -5°C in an ice bath, add 70.4g of aluminum trichloride in four times, and keep the temperature at -5- 0°C, stirring and reacting for 5 hours, the reaction solution was treated according to conventional methods to obtain 76.8g of 3-(4-chlorobutyryl)indole-5-carbonitrile; the obtained intermediate was added with 800ml of diethyl ether, cooled to -5°C in an ice bath, and divided into four Add 30 g of sodium borohydride each time, add 150 g of trifluoroacetic acid dropwise after the addition, keep warm at -5-0°C and stir for 5 hours after the addition, after the reaction is completed, post-process according to the conventional method to obtain 3-(4-chlorobutyl)indole-5- Amethonitrile was 60.6g, the total yield was 74%, and the HPLC purity was 99.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com