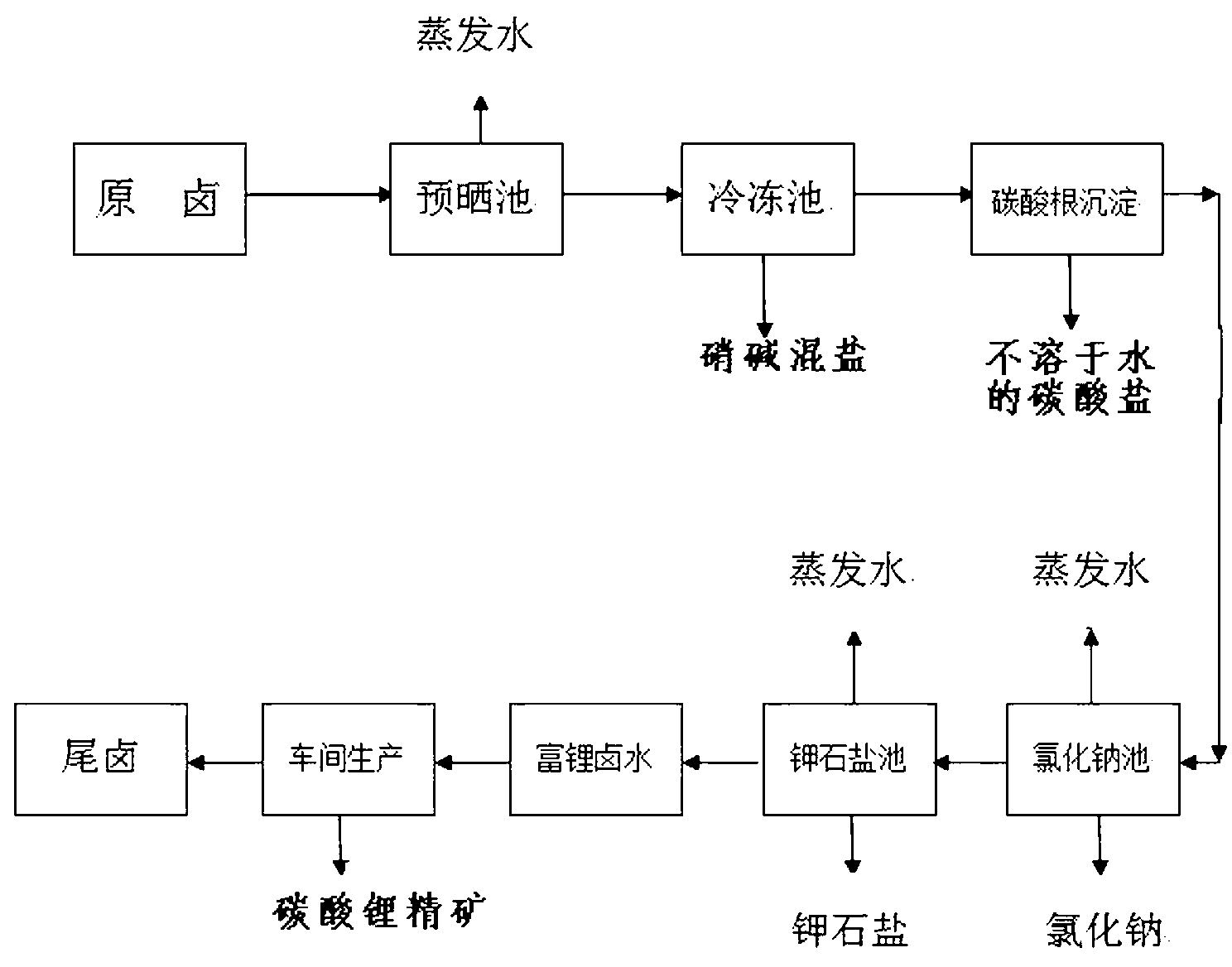
Method for separating carbonate from carbonate bittern containing lithium and potassium to prepare sylvinite ore and lithium carbonate concentrate
A carbonate type, potassium rock salt mine technology, applied in the direction of lithium carbonate;/acid carbonate, alkali metal chloride, etc., can solve the problem of long production cycle, unfavorable potassium recycling, low lithium carbonate content and other problems, to achieve the effect of easy control, continuous industrialization and automatic production, and continuous and stable production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1: Take 600kg of raw brine from Dangxiong Co Salt Lake (see Table 13 for the chemical composition of the brine), the lithium content in the original brine is 0.32g / l, evaporate to a brine density of 1.21g / ml, and a lithium content of 0.76g / l to prepare Get 140.01kg of pre-dried brine. Evaporate until the brine has a density of 1.26g / ml and a lithium content of 0.94g / l to obtain 116.037kg of pre-dried brine, whose chemical composition (unit: g / L) is shown in Table 2.
[0038] Table 2: Chemical composition of pre-dried brine
[0039]
[0040] Table 13: Dangxiong Co Salt Lake brine chemical composition (sampling time: 2012.3.28, analysis time: 2012.4.1)
[0041]
Embodiment 2
[0042] Example 2: Divide 140.01 kg of pre-dried brine with a density of 1.21 g / ml into 2 parts, each 70 kg. The 116.037kg pre-sunned brine with a density of 1.26g / ml was divided into 3 parts of 38.679kg each for freezing alkali, and the hydration of sodium carbonate and sodium sulfate was frozen at -5°C, -15°C, and -25°C respectively For mixed salt, its quantity is shown in Table 3, and its chemical composition is shown in Table 4;
[0043] product number k + Na + CL - SO 4 2- CO 3 2- B 2 o 3 Li + Pre 1 25.40 123.6 163.7 14.76 23.597 6.766 0.76 pre 2 27.10 125.4 165.6 16.21 28.844 8.575 0.940 Nitrate 1 1.192 35.745 6.130 37.423 19.127 0.101 0.041 Nitrate 2 1.598 36.850 3.787 26.477 28.670 2.560 0.044 Nitrine 3 1.364 36.874 19.070 22.910 18.166 1.462 0.018 Nitrine 4 1.494 37.544 19.799 13.353 23.451 4.133 0.043 Nitrine 5 2.682 36.641 22.299 12.723 21.589 3.883 ...
Embodiment 3
[0057] Embodiment 3: 31.854 kg of bittern after desulfurization and carbonate radicals are introduced into the sodium chloride pond to naturally evaporate and separate out 3.921 kg of halite, and its chemical dry basis composition is (unit %) shown in Table 7: obtain potassium saturated bittern 16.917 kg simultaneously, At this time, the potassium ion content in the brine is 51.700 g / L, and its chemical composition (unit g / L) is shown in Table VII.
[0058] Material name k + Na + CL - SO 4 2- CO 3 2- B 2 o 3 Li + Precipitated halite 0.973 38.576 60.011 0.028 0.000 0.160 0.034 Potassium saturated brine 51.700 97.660 189.900 1.500 0.00 8.510 1.810
[0059] Embodiment 3 is a sodium chloride sun-drying process: generally, sodium chloride is separated out by natural evaporation and crystallization, and sodium chloride is separated;
PUM
| Property | Measurement | Unit |
|---|---|---|
| Density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com