Preparation method and application of sulfur and phosphorus composite artificial bone
A composite, artificial bone technology, applied in medical science, prosthesis, etc., can solve the problem of the mismatch between the rate of degradation and the rate of new bone formation, and achieve the effect of solving the mismatch between the rate of degradation and the rate of new bone formation and accelerating repair.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
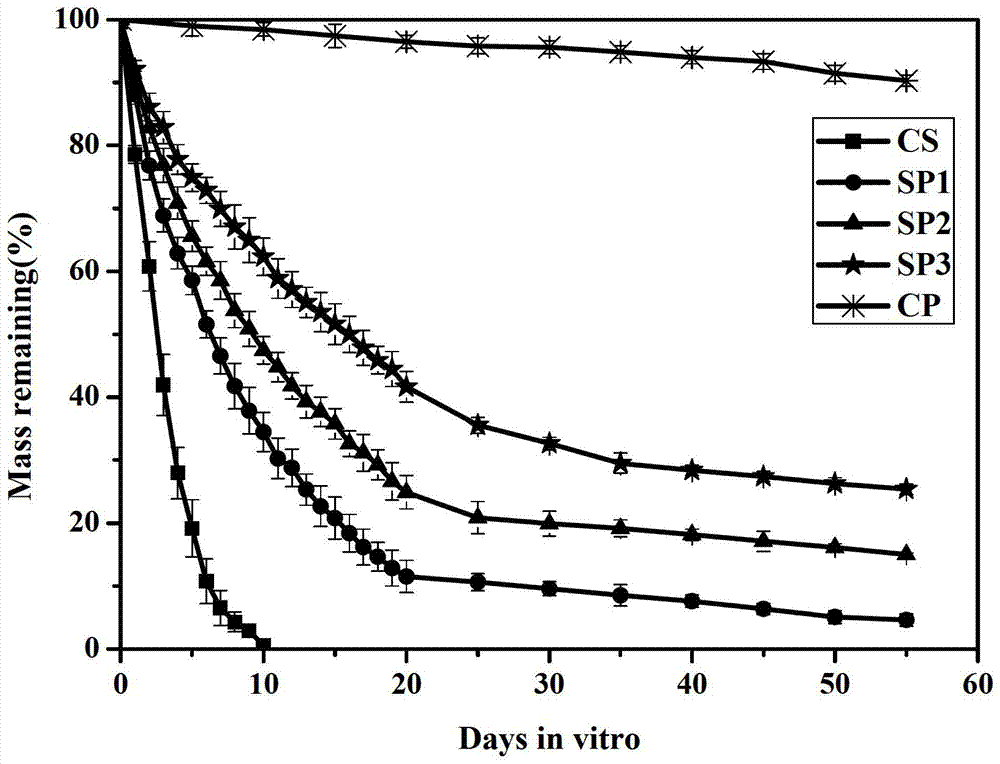
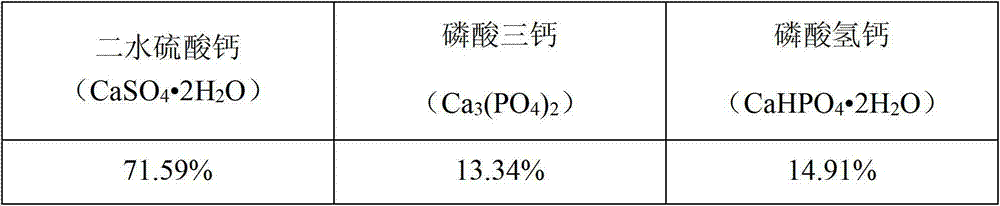
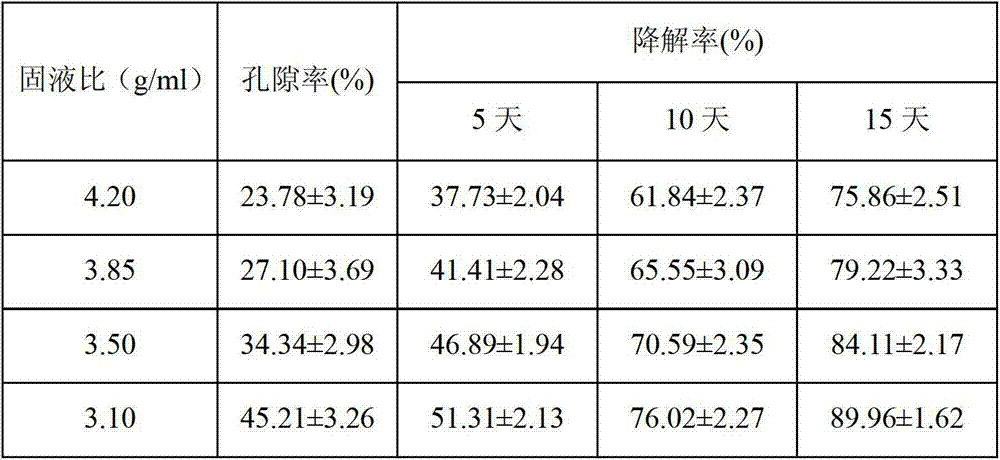
Image
Examples
Embodiment 1
[0030] According to the chemical composition requirements of the sulfur-phosphorus composite artificial bone, a certain amount of calcium sulfate hemihydrate, tricalcium phosphate, and calcium dihydrogen phosphate were weighed to form solid ingredients. Among them, tricalcium phosphate accounts for 22.5% of the total mass of solid ingredients, and the particle size of tricalcium phosphate is (100~600) μm; calcium sulfate hemihydrate accounts for 70% of the total mass of solid ingredients, and the particle size of calcium sulfate hemihydrate is (10~100) μm; calcium dihydrogen phosphate accounts for 7.5% of the total mass of solid ingredients, and the particle size of calcium dihydrogen phosphate is (50~300) μm. Pour the solid ingredients into the v-shaped barrel mixer and mix for 40 minutes at a rate of 20r / min. Then pour the mixed material into the kneader, add 130ml of normal saline, and stir and knead for 120s. Take out the kneading material and pour it on the rubber templa...
Embodiment 2
[0032] According to the chemical composition requirements of the sulfur-phosphorus composite artificial bone, a certain amount of calcium sulfate hemihydrate, tricalcium phosphate, and calcium dihydrogen phosphate were weighed to form solid ingredients. Among them, tricalcium phosphate accounts for 37.5% of the total mass of solid ingredients, and the particle size of tricalcium phosphate is (100~600) μm; calcium sulfate hemihydrate accounts for 50% of the total mass of solid ingredients, and the particle size of calcium sulfate hemihydrate is (10~100) μm; calcium dihydrogen phosphate accounts for 12.5% of the total mass of solid ingredients, and the particle size of calcium dihydrogen phosphate is (50~300) μm. Pour the solid ingredients into the v-shaped barrel mixer and mix for 40 minutes at a rate of 20r / min. Then pour the mixed material into the kneader, add 130ml of physiological saline, and stir and knead for 150s. Take out the kneading material and pour it on the rub...
Embodiment 3
[0034]According to the chemical composition requirements of the sulfur-phosphorus composite artificial bone, a certain amount of calcium sulfate hemihydrate, tricalcium phosphate, and calcium dihydrogen phosphate were weighed to form solid ingredients. Among them, tricalcium phosphate accounts for 52.5% of the total mass of solid ingredients, and the particle size of tricalcium phosphate is (100~600) μm; calcium sulfate hemihydrate accounts for 30% of the total mass of solid ingredients, and the particle size of calcium sulfate hemihydrate is It is (10~100) μm; calcium dihydrogen phosphate accounts for 17.5% of the total mass of solid ingredients, and the particle size of calcium dihydrogen phosphate is (50~300) μm. Pour the solid ingredients into the v-shaped barrel mixer and mix for 40 minutes at a rate of 20r / min. Then pour the mixed material into the kneader, add 130ml of physiological saline, and stir and knead for 150s. Take out the kneading material and pour it on the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com