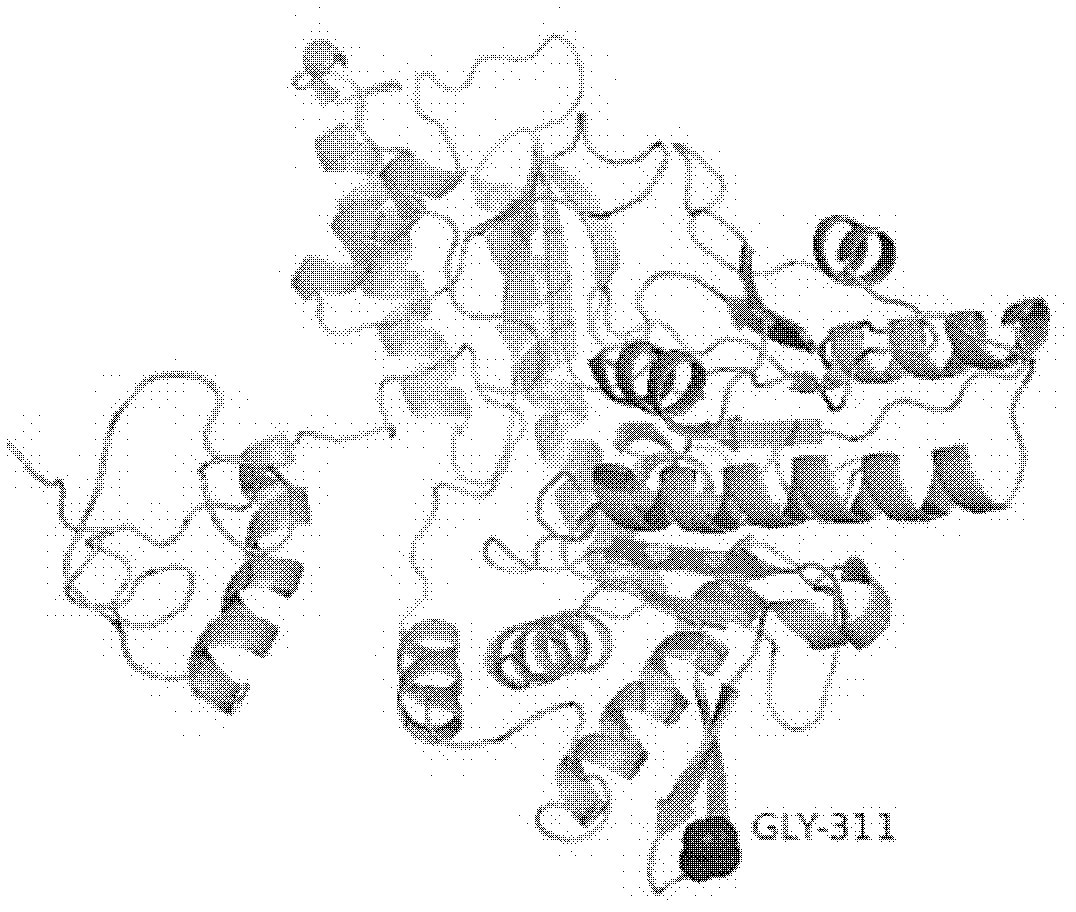
Glutamate decarboxylase (GAD) thermally-stable variant G311P gene and application thereof
A technology of glutamic acid decarboxylase and thermal stability, which is applied in the field of molecular biology and can solve problems such as the application of unfavorable enzymes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0020] For further illustrating the present invention, specifically illustrate in conjunction with following examples:
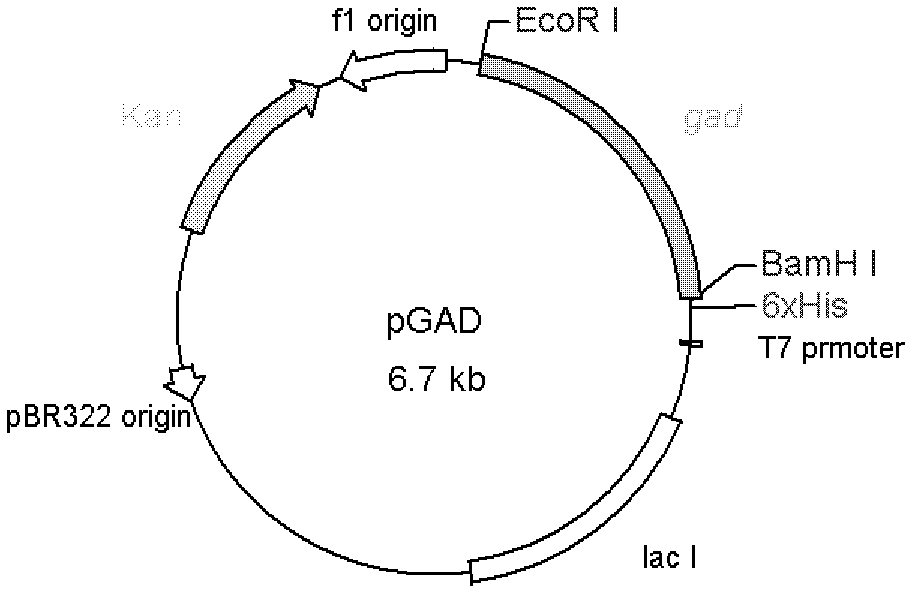
[0021] 1. Construction of recombinant plasmids
[0022] Lactobacillus brevis (Lactobacillus brevis) CGMCC NO.1306 was cultivated to the mid-logarithmic growth phase, 4.5 mL of the bacterial liquid was centrifuged at 10,000 rpm for one minute, the supernatant was discarded, and genomic DNA was extracted using a kit (Shanghai Sangong) according to the instructions.
[0023] Design the following degenerate primers:
[0024] Upstream degenerate primer: 5′-cg ggatcc atggcWatgttRtaYggWaaac-3' (as shown in SEQ ID NO.5);
[0025] Downstream degenerate primer: 5′-gg gaattc ttagtgHgtgaaYccgtattt-3' (as shown in SEQ ID NO.6);
[0026] Wherein, "ggatcc" in the upstream primer is the BamH I restriction site, and "gaattc" in the downstream primer is the EcoR I restriction site.
[0027] Use upstream primers and downstream primers to pair up, select and optimize dif...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com