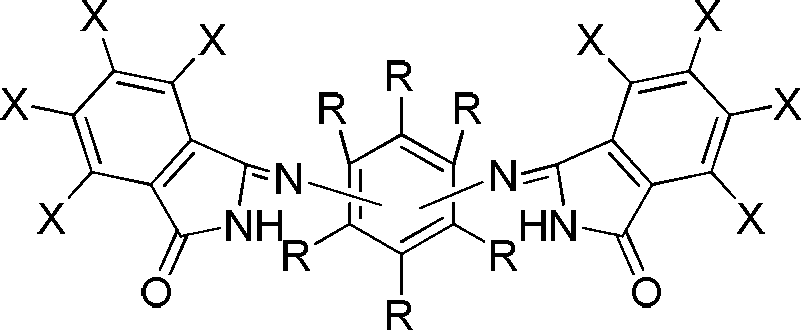
Process for synthesizing isoindolinone pigment under mild conditions
A technology of isoindolinone and synthesis process, applied in the direction of organic dyes, etc., can solve the problems of harsh conditions, complicated operation, high energy consumption, etc., and achieve the effect of mild reaction conditions and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] 30% sodium methylate solution and 202L methyl alcohol of 34.5Kg of dropping into 34.5Kg, 6-tetrachloro-o-cyanobenzoic acid methyl ester, 27Kg and 202L methyl alcohol were kept at room temperature for 1 hour, then added 5.8Kg of The phenylenediamine was refluxed for 3 hours, and then 10L of water was added, at this time, the pigment precipitated out and was bright yellow. After filtering, washing with 200L of methanol and 200Kg of water, vacuum-dried to obtain 34Kg of pigment.
Embodiment 2
[0023] Put 69Kg of 3,4,5,6-tetrachloro-o-cyanobenzoic acid methyl ester, 68Kg of 30% sodium ethoxide solution and 424L ethanol into the reaction kettle and keep it at room temperature for 1 hour, then add 11.6Kg of m-benzene The diamine was refluxed for 4 hours, and then 30L of water was added, at this time, the pigment precipitated out, showing bright green light yellow. After filtering, washing with 500L of methanol and 500Kg of water, vacuum-dried to obtain 68Kg of pigment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com