Polyethylene glycol benzothiazole derivative and preparation method and application thereof
A polyethylene glycol and benzothiazole technology, applied in the field of polyethylene glycol benzothiazole derivatives and its preparation, can solve the problems of PEG-MAL instability and large difference in biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0028] Reagents and Instruments
[0029] Reagents: dichloromethane (DCM), tetrahydrofuran (THF), diethyl ether (DEE), all of which are of analytical grade except those used for determination; N-methylmorpholine (4-methylmorpholine, NMP) , Isobutyl chloroformate (isobutyl chloroformate, ClCOOBu i ), tert-Butylcarbonyl glycine (Boc-gly), dicyclohexylcarbodiimide (DCC), 4-dimethylaminopyridine (4-dimethylaminopyridine, DMAP), crystal violet And phosphate etc. come from Sinopharm Chemical Reagent Company; 2-cyano-6-aminobenzothiazole (2-cyano-6-amidebenzothiazole, CBT-NH 2), cysteine (Cysteine, Cys), homocysteine (Homocysteine, Hcy), newborn bovine serum (fetal bovine serum, FBS), thiazolyl blue (MTT) were derived from Sigma-Aldrich; bovine serum albumin (Solarbio); RPMI1640 culture fluid (GbicoBR1); Polypeptide CT20 (CTFFYGGSRGKRNNFKTEEY) comes from Shanghai Qiangyao Biological Company; N-terminal cysteine interferon a-2b (N-Cys-IFN, purified in this laboratory); Ethylen...
Embodiment 1
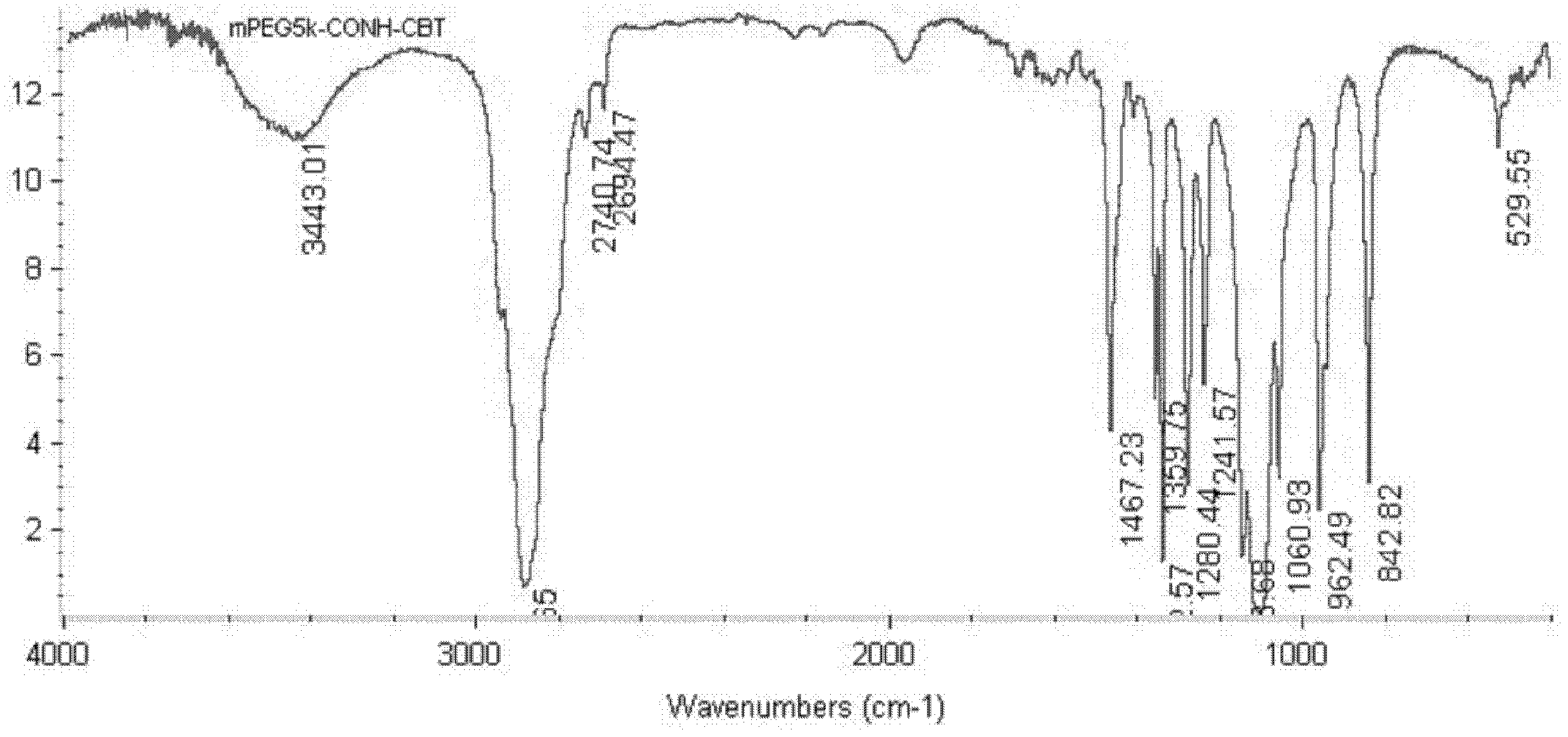
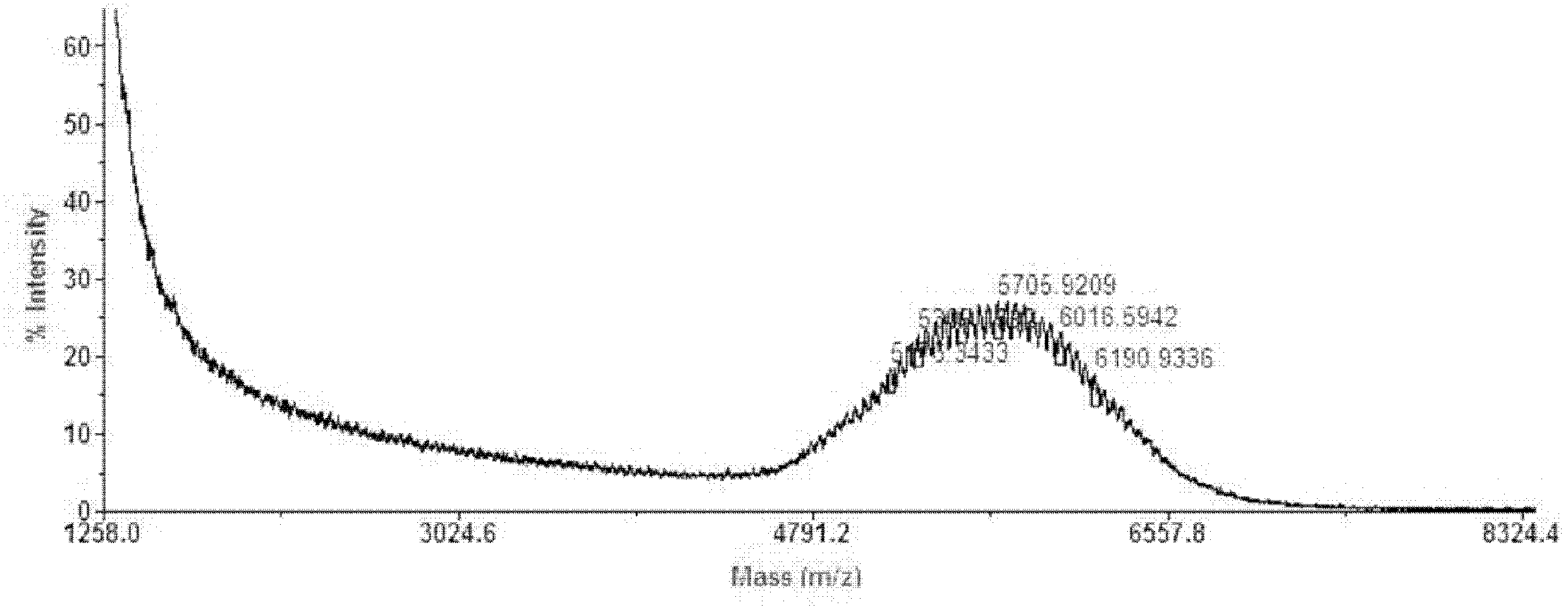
[0031] Example 1. Preparation of MePEG 5k -CONH-CBT
[0032] Get monomethoxy terminal carboxyl polyethylene glycol 5000 10g (MePEG 5k -COOH, 2.0mmol) was dissolved in DCM 50ml solution, filled with N at 0°C 2 Under the condition of stirring for 10min, add ClCOOBu i 0.410g (3mmol), NMP 0.40g (4mmol), the above mixture was stirred and reacted for 20min; 2 0.7g (4mmol) of THF 50ml solution, reacted under the above conditions for 2h, then moved to room temperature to continue the reaction for 24h; vacuum rotation to remove the organic solvent, dissolved the residue in brine, filtered off the insoluble matter, extracted with DCM, concentrated the organic phase, and precipitated with anhydrous ether Concentrate the solution and wash the precipitate several times to get MePEG after removing the ether 5k -CONH-CBT 9.5g.
[0033]
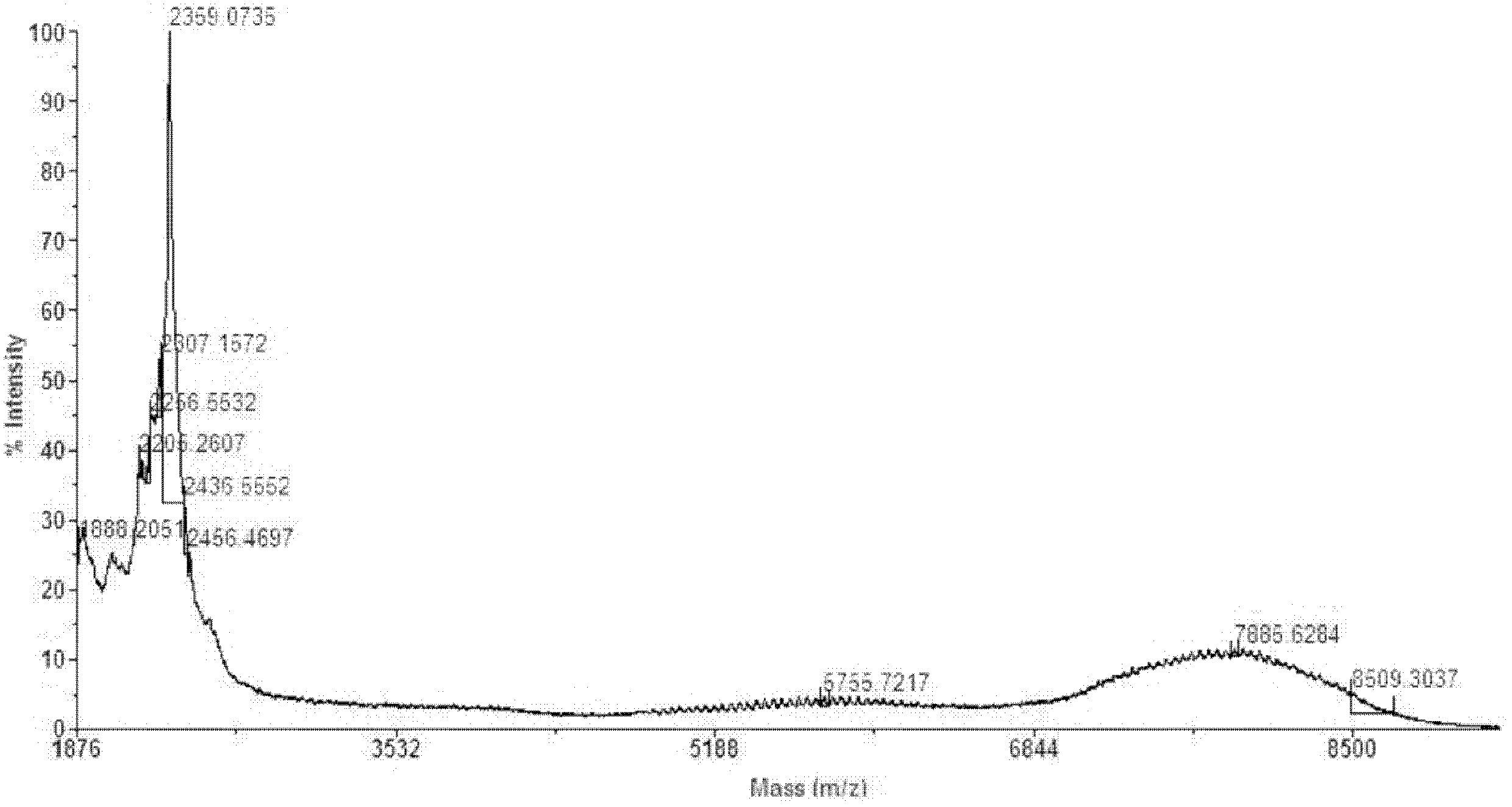
Embodiment 2
[0034] Example 2. Preparation of CBT-NHCO-PEG 5k -CONH-CBT
[0035] Get two carboxyl polyethylene glycol 5000 10g (HOOC-PEG 5k -COOH, 2.0mmol) was dissolved in DCM 50ml solution, filled with N at 0°C 2 Under the condition of stirring for 10min, add ClCOOBu i 0.820g (6mmol), NMP 0.80g (8mmol), the above mixture was stirred and reacted for 20min; 2 1.4g (8mmol) THF 50ml solution, after reacting for 2h under the above conditions, move to room temperature and continue to react for 24h; under vacuum condition, rotary evaporation removes organic solvent, dissolves residue in brine, filters out insoluble matter, extracts with DCM, concentrates organic phase, no Precipitate the concentrated solution with water and ether and wash the precipitate several times to obtain CBT-NHCO-PEG after removing the ether 5k -CONH-CBT 9.75g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com