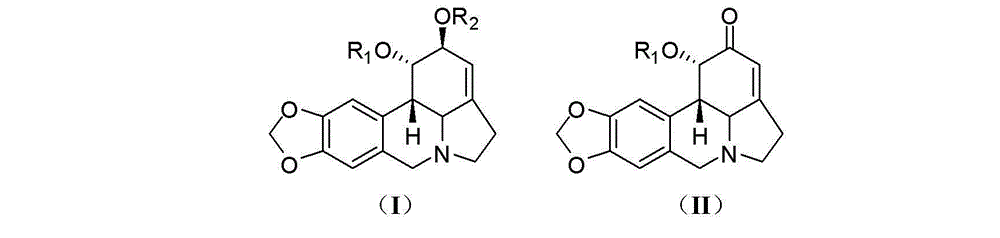
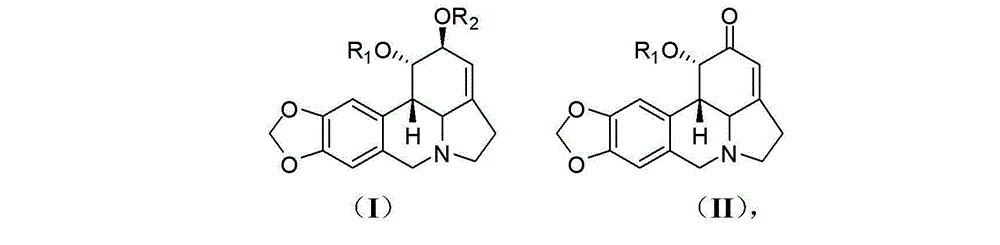
Lycorine derivative, veterinary drug taking lycorine derivative as an active constituent, and applications of lycorine derivative
A technology of lycorine and veterinary medicine, which is applied in the field of preparation of veterinary medicine for treating porcine reproductive and respiratory syndrome (porcine blue ear disease), can solve problems such as inability to provide effective protection for pig farms, and achieve remarkable curative effect, economical applicability, Effects in Moderate Dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Preparation of compound 1-O-(methylthio)methyl-2-O-acetyllycorine (KY-91): Take 50 mg (0.152 mmol) of 2-O-acetyllycorine and add dimethyl sulfoxide ( 0.5 mL) and acetic anhydride (0.35 mL), and stirred overnight at room temperature. The reaction system was extracted by adding water (0.5 mL) and ammonia water (0.5 mL), then extracted with ether, evaporated to dryness, separated by silica gel column chromatography, and eluted with petroleum ether-ethyl acetate (4?1) to obtain Compound KY-91 (15 mg, 0.0384 mmol, 25%).
[0029] .
[0030] Compound KY-91 is light yellow solid; 1 H-NMR (CD 3 OD, 400 MHz): δ 7.09 (s, 1H, H-8), 6.62 (s, 1H, H-12), 5.90 (s, 2H, H 2 -11), 5.75 (br s, 1H, H-2), 5.49 (br s, 1H, H-3), 4.70 and 4.65 (d, J = 12.0 Hz, 1H each, OCH 2 SCH 3 ), 4.57 (br s, 1H, H-1), 4.12 and 3.52 (d, J = 14.0 Hz, 1H each, H 2 -7), 3.46 and 2.44 (m, 1H each, H 2 -5), 2.84 (d, J = 10.4 Hz, 1H, H-12b), 2.72 (d, J = 10.4 Hz, 1H, H-12c), 2.66 (m, 2H, H 2 -4), 2.17 (...
Embodiment 2
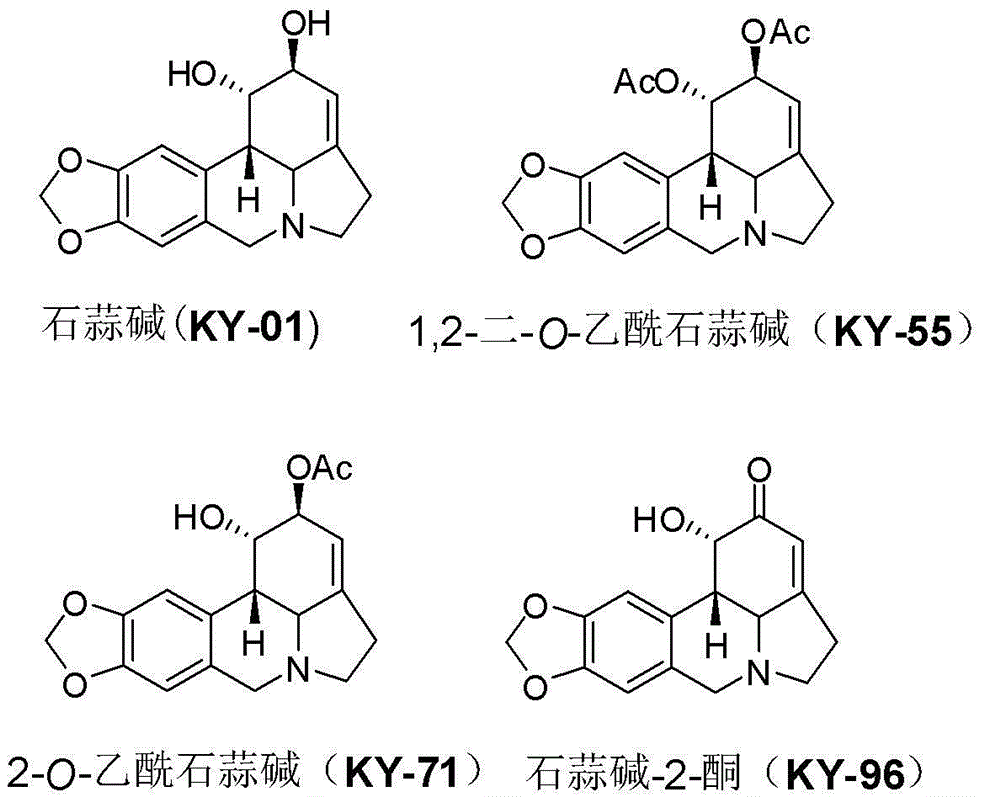
[0032] Compounds Lycorine (KY-01) [Wang et al. Chin Herb Med 2011, 3: 60-63], 1,2-di-O-acetyllycorine (KY-55) [Lee et al. Bioorg Med Chem 2007, 15 (2), 1034-1043], 2-O-acetyllycorine (KY-71) [Toriizuka et al. Bioorg Med Chem 2008, 16 (24), 10182-10189] and lycorine- 2-Keto (KY-96) [Wang et al. Chem Cent J 2012, 6: 96] was prepared according to the method reported in the literature.
[0033] Lycorisine (KY-01) preparation method: extract the bulb and flower of Lycoris radiata (Lycoris radiata) with methanol, recover the solvent to obtain the extract, add water to make a suspension, extract with petroleum ether, and use the water phase Adjust the pH to about 2 with 1% hydrochloric acid and extract with ethyl acetate. The aqueous phase was adjusted to a pH value of about 10 with 5% sodium hydroxide, extracted with chloroform, and the solvent was recovered to obtain a chloroform extract. The chloroform extract was subjected to column chromatography on C-18 silica gel, methanol-w...
Embodiment 3
[0045] According to the method of Example 1 or 2, lycorine and its derivatives are first prepared, and water for injection is added as usual, finely filtered, potted and sterilized to make an injection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com