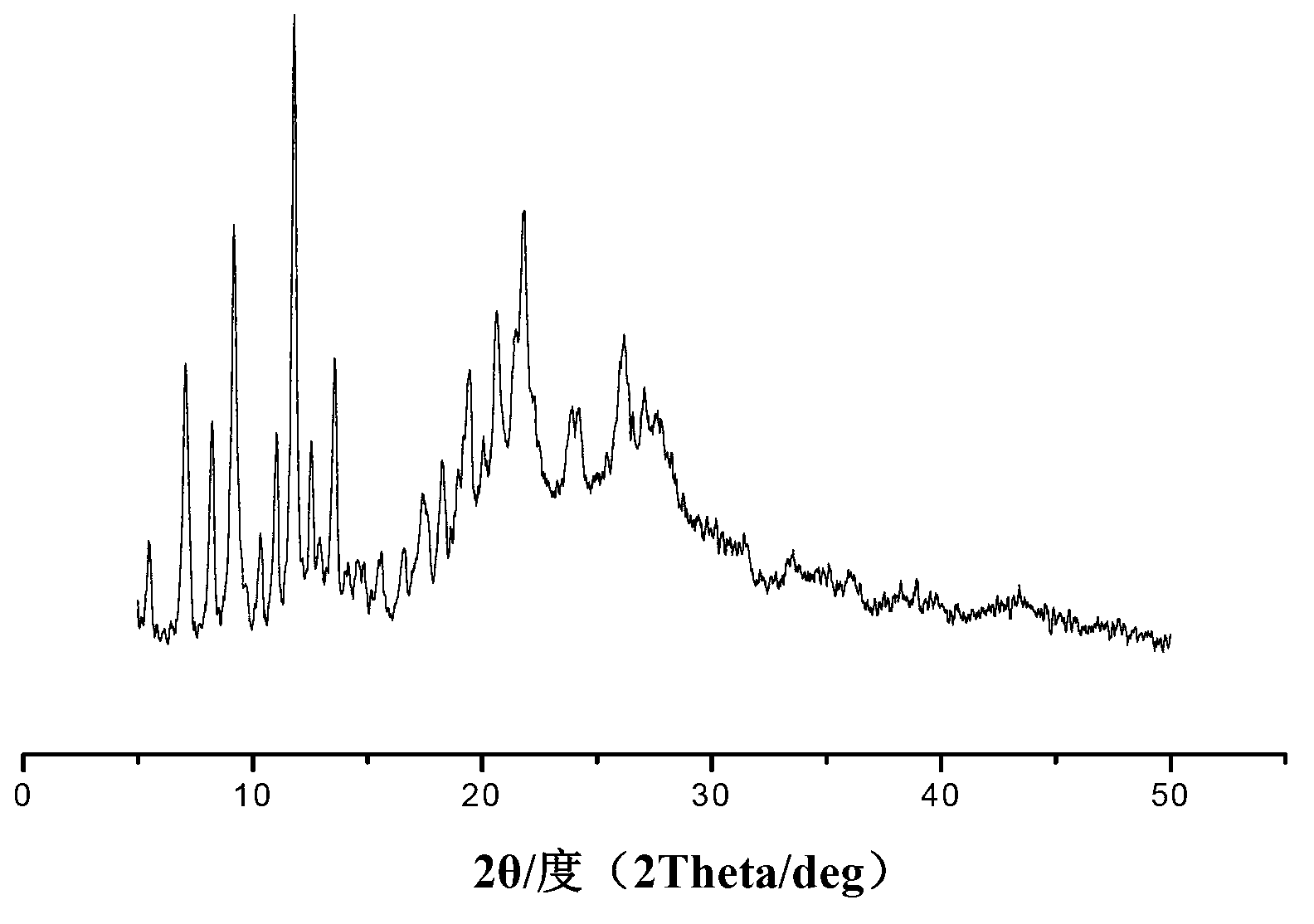
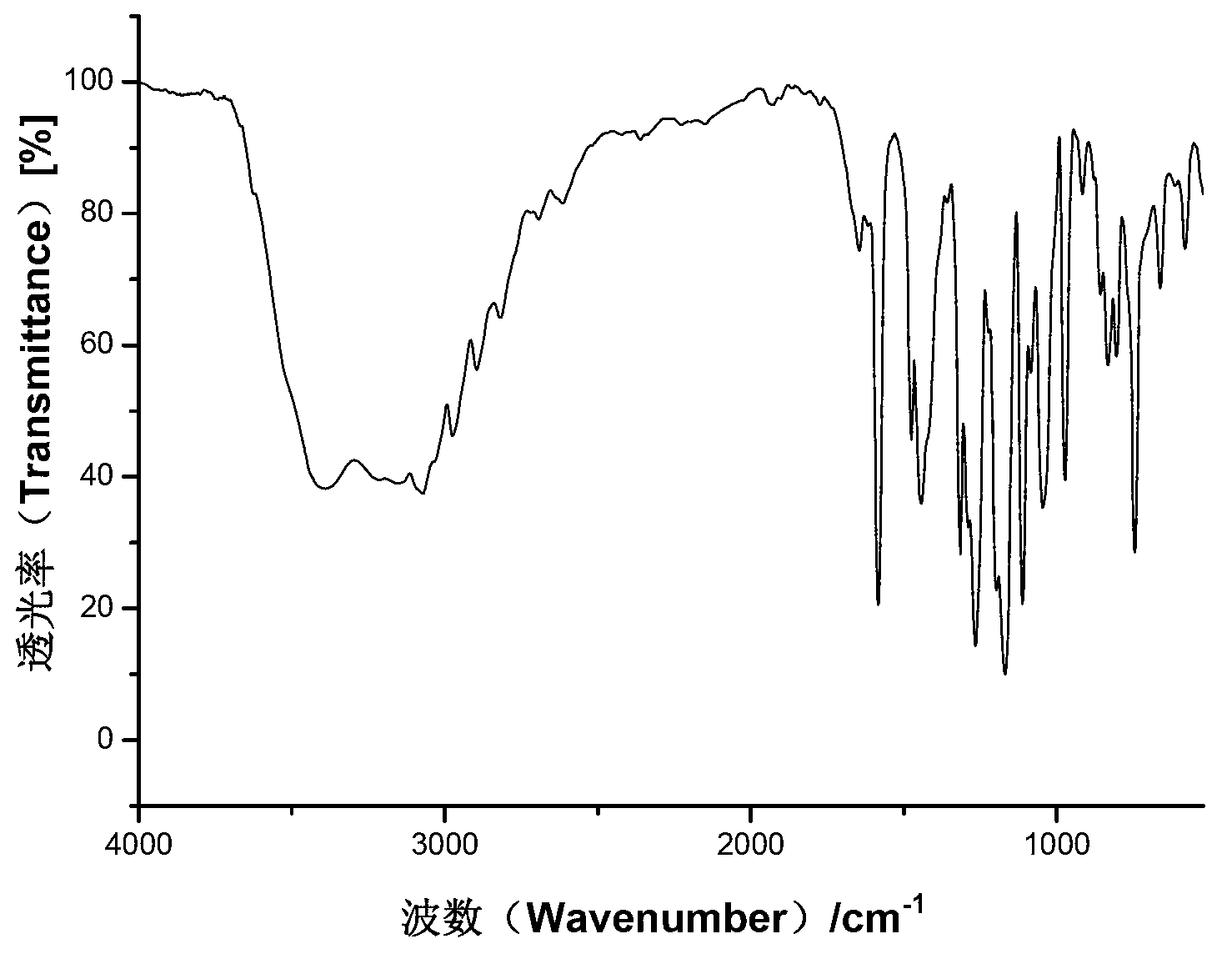
Lansoprazole N crystal form and preparation method and application thereof
A technology of lansoprazole and crystal form, applied in the field of new crystal form of lansoprazole and its preparation, can solve the problems of complicated preparation process, time-consuming and the like, and achieves simple operation, less solvent and good reproducibility Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0028] The preparation method of Lansoprazole N crystal form, comprises the following steps:
[0029] 1) Lansoprazole is dissolved in ethyl acetate, or n-butanol and water volume ratio are in the mixed solvent of 10:1;
[0030] 2) Cool the above solution to -20~-10°C to precipitate crystals;
[0031] 3) The crystals are filtered and dried to obtain the N crystal form of lansoprazole.
Embodiment 1
[0035] 1) 50mg lansoprazole was dissolved in 0.1mL ethyl acetate, filtered;
[0036] 2) Cool down the clear solution to -10°C to make it fully crystallized, filter and dry.
[0037] Obtained 42.7 mg of off-white crystalline powder with a yield of 85.4%.
Embodiment 2
[0039] 1) 100mg lansoprazole was dissolved in 0.5mL ethyl acetate, filtered;
[0040] 2) Cool down the clear solution to -10°C to make it fully crystallized, filter and dry.
[0041] 79.6 mg of off-white crystalline powder was obtained, with a yield of 79.6%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com