Novel preparation method for ivabradine
A compound and hydrogenation reaction technology, applied in the field of medicine and chemical industry, can solve the problems of complicated preparation operation of compound I, unsuitable for industrial scale-up production, low yield of docking reaction, etc., achieve good industrialization basis and application value, reduce synthesis difficulty, The effect of high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
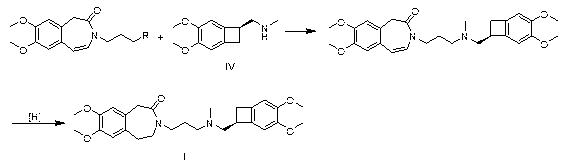
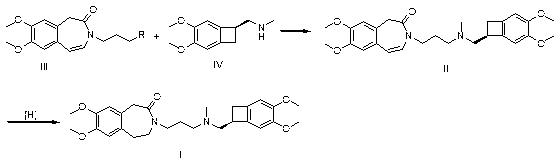
[0029] Take 2.96g of compound III, wherein R is Cl, 2.44g of compound IV, 1.38g of potassium carbonate and 1.50g of sodium iodide in 50mL of methyl isobutyl ketone for reflux reaction for 6h, cool, add 50mL of 1N hydrochloric acid, separate layers, Collect the aqueous layer, adjust the aqueous layer to pH = 10 with sodium hydroxide, extract with ethyl acetate, dry over anhydrous sodium sulfate, filter with suction, and concentrate the filtrate to dryness to obtain 4.5 g of the intermediate, namely 7,8-dimethoxy -3-(3-[[(1S)(4,5-dimethoxybenzocyclobutan-1-yl)methyl]-methylamino]propyl)-1,3,-dihydrogenated-2 Hydrogen-benzazepin-2-one;
[0030] In a 50 mL reaction flask, add 2.0 g 7,8-dimethoxy-3-(3-[[(1S)(4,5-dimethoxybenzocyclobutane-1-yl)methyl ]-methylamino]propyl)-1,3,-dihydro-2hydro-benzazepin-2-one, 20 mL of methanol, 0.3 g of ammonium formate, 0.2 g of palladium on carbon, hydrogenation reaction pressure of 1 atm, Raise the temperature to 30°C and stir for 4 hours, filt...
Embodiment 2
[0032] Take 3.40g of compound III, where R is Br, 2.44g of compound IV, 0.69g of potassium carbonate and 1.50g of potassium iodide in 50mL of acetone for 12h under reflux, filter, concentrate the filtrate, add 50mL of 1N hydrochloric acid to the residue, and wash with sodium hydroxide Adjust to pH=9, extract with ethyl acetate, dry over anhydrous sodium sulfate, filter with suction, and concentrate the filtrate to dryness to obtain 4.6g of intermediate, namely 7,8-dimethoxy-3-(3-[[(1S )(4,5-dimethoxybenzocyclobutan-1-yl)methyl]-methylamino]propyl)-1,3,-dihydro-2hydro-benzazepine-2- ketone;
[0033] In a 50 mL reaction flask, add 2.0 g 7,8-dimethoxy-3-(3-[[(1S)(4,5-dimethoxybenzocyclobutane-1-yl)methyl ]-methylamino]propyl)-1,3,-dihydro-2hydro-benzazepin-2-one, 20 mL ethanol, 0.6 g ammonium formate, 0.5 g palladium carbon, hydrogenation reaction pressure is 3 atm, Raise the temperature to 60°C and stir for 5 hours, filter, and concentrate the filtrate to dryness to obtain 1.9...
Embodiment 3
[0035] Take 3.86g of compound III, where R is I, 2.44g of compound IV, 0.69g of potassium carbonate and 1.50g of potassium iodide in 50mL of methyl ethyl ketone for 12h under reflux, filter, concentrate the filtrate, add 50mL of 1N hydrochloric acid to the residue, and Adjust the pH to 11 with sodium, extract with ethyl acetate, dry over anhydrous sodium sulfate, filter with suction, and concentrate the filtrate to dryness to obtain 4.4 g of the intermediate, namely 7,8-dimethoxy-3-(3-[[( 1S)(4,5-dimethoxybenzocyclobutan-1-yl)methyl]-methylamino]propyl)-1,3,-dihydro-2hydro-benzazepine-2 -ketone;
[0036] In a 50 mL reaction flask, add 2.3g 7,8-dimethoxy-3-(3-[[(1S)(4,5-dimethoxybenzocyclobutane-1-yl)methyl ]-methylamino]propyl)-1,3,-dihydro-2hydro-benzazepine-2-one, 23 mL ethanol, 3.1 g ammonium formate, 2.3 g palladium carbon, hydrogenation reaction pressure is 1 atm, Raise the temperature to 80°C and stir for 1.5 hours, filter, and concentrate the filtrate to dryness to ob...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com