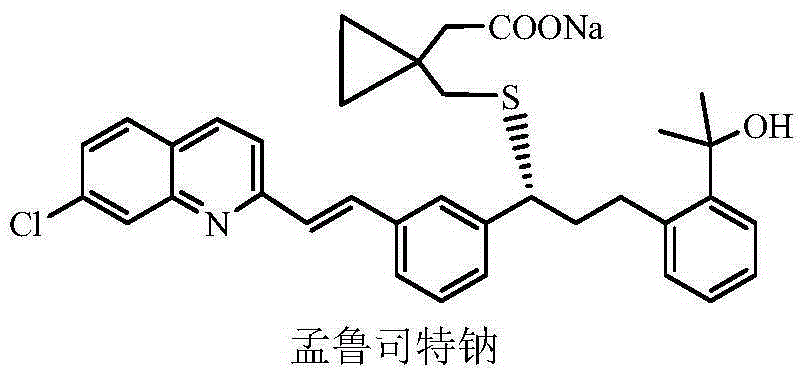
A kind of preparation method of montelukast sodium and its intermediate
A technology of montelukast sodium and montelukast acid, which is applied in the field of synthesis of montelukast sodium and its intermediates, can solve the problems of low overall yield and increase purification procedures, and achieve mild and easy-to-control reaction conditions , Simple process operation, good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1 Preparation of compound 2-(3-(3-(2-(7-chloro-2-quinolyl) vinyl) phenyl)-3-oxopropyl) phenyl)-2-propoxy) Tetrahydropyran (Intermediate 2)
[0040] According to the patent CN101638381, the total yield is 41%.
Embodiment 2
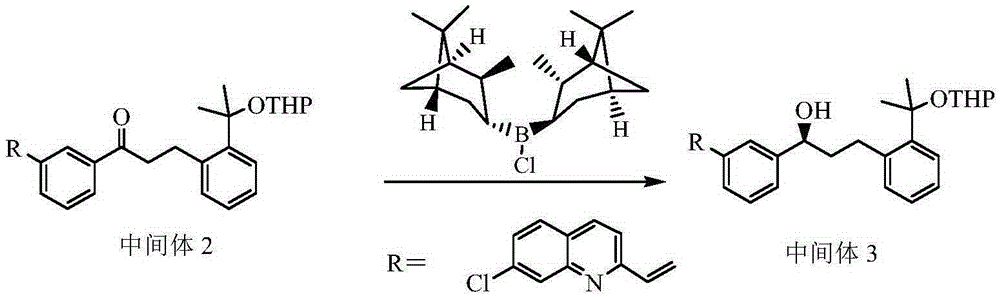
[0041] Example 2 Preparation of Compound 2-(3(S)-(3-(2-(7-chloro-2-quinolyl)vinyl)phenyl)-3-hydroxypropyl)phenyl)-2-propane oxy)tetrahydropyran (Intermediate 3)
[0042] Dissolve 115.0g (0.359mol) of (-)-diisopine pinocampyl chloride borane in 480ml of THF, and cool the solution to -15°C; dissolve 129.1g (0.239mol) of Intermediate 2 in 560ml of THF, Add this solution dropwise to the above-mentioned (-)-diisopinepinene chloroborane solution at -15°C to obtain a red solution. After dropping, slowly raise the temperature to 15°C while stirring, and react at this temperature for 6 hours. Poured into ice water, the reaction solution precipitated a solid precipitate, suction filtered, washed the solid twice with water and once with ethyl acetate; added the solid to 1.9L of 6% diethanolamine aqueous solution, then added 4L of dichloromethane, stirred for 10 Minutes later, the liquid was separated, and the aqueous phase was extracted once more with dichloromethane, and the dichlorome...
Embodiment 3
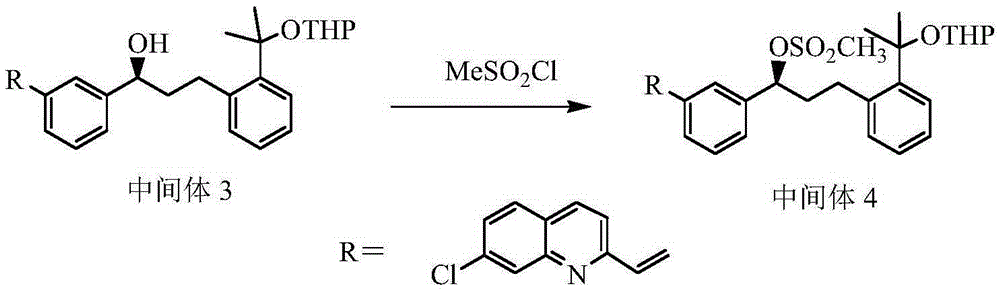
[0043] Example 3 Preparation of compound 2-(3(S)-(3-(2-(7-chloro-2-quinolyl)vinyl)phenyl)-3-methylsulfonylpropyl)phenyl)-2 -propoxy)tetrahydropyran (intermediate 4)
[0044] Dissolve 43.0g (79.4mmol) of intermediate 3 in 800ml of dichloromethane, and cool to -10°C while stirring; add 11.82g (103mmol) of methanesulfonyl chloride and 15.4g (119mmol) of triethylamine into the reaction solution , continue to stir at -10°C for 30 minutes, then slowly heat up to 0°C, and react for another 1h. After the reaction is complete, add 400ml of saturated sodium bicarbonate solution, stir for 10 minutes and then separate the liquids. The aqueous phase is extracted with dichloromethane (200ml× 2), combined extracts, anhydrous Na 2 SO 4 Drying; filtering, concentration, the residue was washed twice with toluene, and dried to obtain 45.1 g of the product with a yield of 91.7%, and the HPLC showed that the purity was 95.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com