Antibody capable of binding to transforming growth factor alpha and having antiproliferative activity on cancer having Ras gene mutation
A ras gene and antibody technology, applied in the direction of transforming growth factor, growth factor/inducer, anti-growth factor immunoglobulin, etc., can solve the problem that there is no effective means for Ras mutant cancer, and achieve the effect of excellent proliferation inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0364] [Example 1] Acquisition of anti-TGFα monoclonal antibody
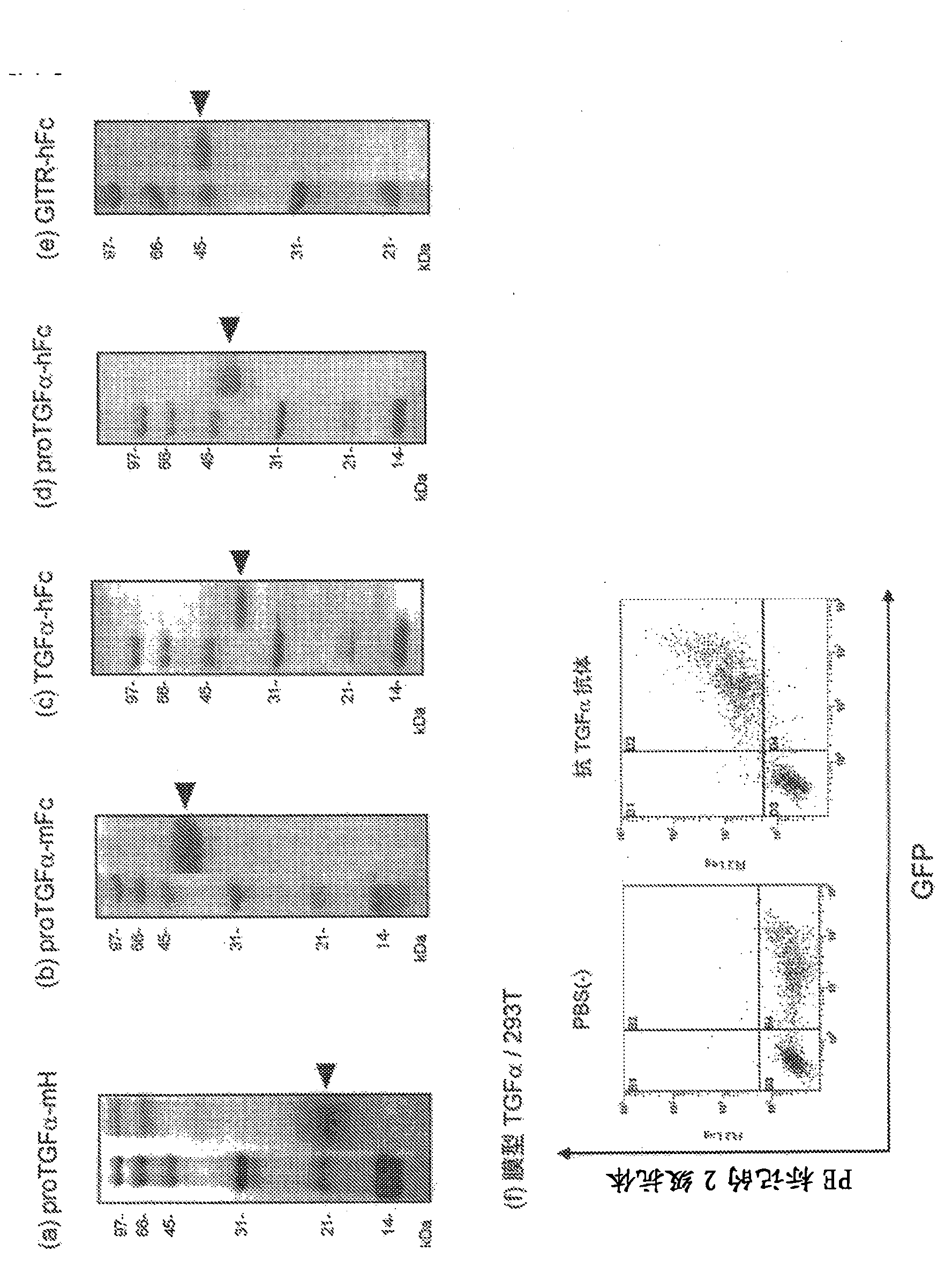
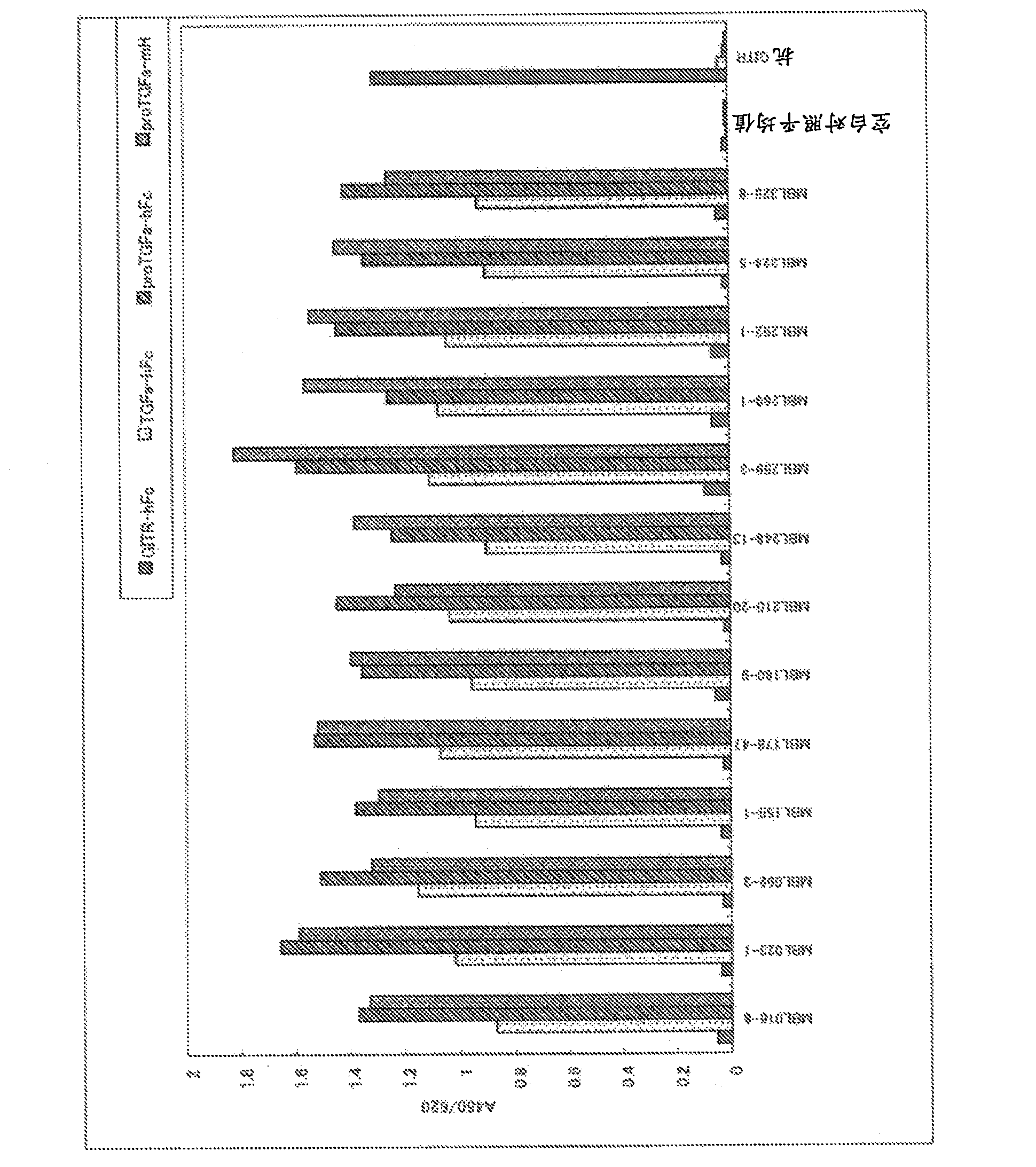
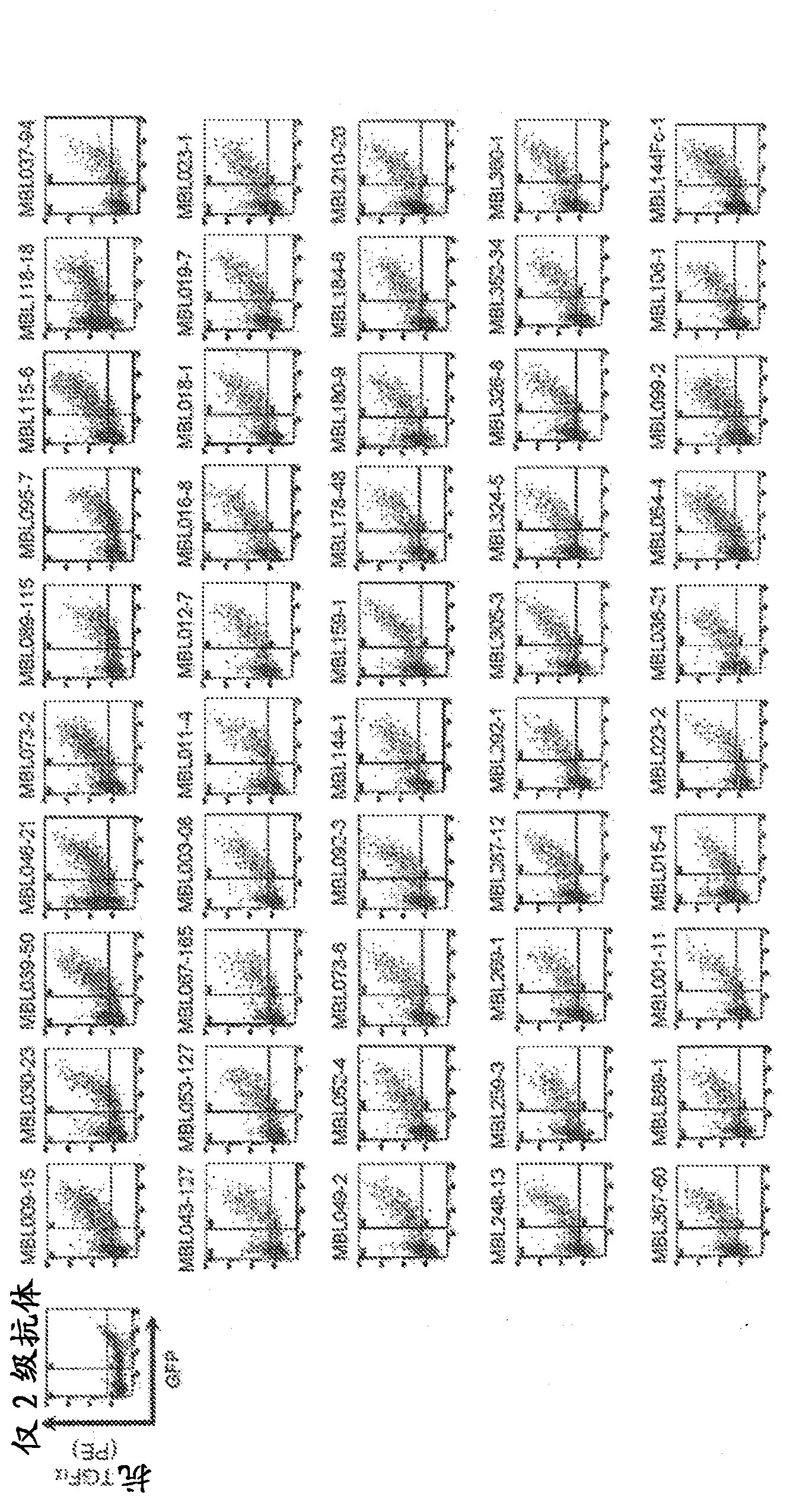
[0365] As the immunogen, proTGFα-mFc alone or proTGFα-mFc and proTGFα-mH were used. A total of 60 mice ( figure 1 a and b). The reactivity with the immunogen or the antigen for screening was evaluated by antigen solid-phase ELISA using hybridoma supernatants fused with lymphocytes and myeloma from immunized mice. Next, TGFα / 293T cells that transiently express the full length of TGFα in 293T cells were used for flow cytometry analysis, and hybridoma supernatants that showed reactivity with membrane-type TGFα were selected, and those capable of immunoprecipitating the secreted antigen proTGFα-mH were selected. Hybridoma supernatant ( Figure 4 ). After the hybridomas were monoclonalized by the limiting dilution method, immunogen solid-phase ELISA and immunoprecipitation were performed again, and a total of 39 anti-TGFα monoclonal antibodies were selected (Table 1). In order to confirm the reactivity of ...
Embodiment 2
[0366] [Example 2] Selection of TGFα neutralizing antibodies that inhibit the binding of TGFα to EGFR
[0367] In order to select an antibody (neutralizing antibody) having the activity of inhibiting the binding of TGFα to EGFR from the obtained anti-TGFα antibody, the following experiment was performed using EGFR highly expressing cancer cell A431. For A431 cultured in serum-free medium, the tyrosine phosphorylation level of EGFR transiently increased when TGFα was added. When A431 was treated after reacting the TGFα solution with each anti-TGFα antibody in advance, an antibody that did not increase the phosphorylation level of EGFR compared with the PBS control was searched. As a result, it was found that MBL352-34, MBL292-1, MBL184-6, MBL016-8, MBL144-1, MBL023-1, and MBL018-1 phosphorylated EGFR tyrosine (P- Tyr1173) inhibits more than 70%-100% ( Figure 5a and b). On the other hand, TGFα neutralizing antibody 189-2130 (Non-Patent Document 50) and EGFR-blocking ant...
Embodiment 3
[0368] [Example 3] Study on membrane-type TGFα or membrane-bound TGFα of cancer cells
[0369] Next, in order to verify the presence of TGFα on the cell surface of cancer cells having a K-Ras gene mutation, flow cytometry analysis was performed using MBL259-3, which has relatively good reactivity to TGFα / 293T cells. As a result, SW620 derived from colorectal cancer, which is a K-Ras gene G12V homomutated cancer cell line, and A427, which is a K-Ras gene G12D heteromutated cell line derived from lung cancer, reacted with MBL259-3 ( Image 6 ).
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com