Pharmaceutical formulation in the form of bilayered tablets comprising HMG-CoA reductase inhibitor and irbesartan
A technology for reductase inhibitors and pharmaceutical preparations, applied in the field of pharmaceutical preparations, can solve the problems of difficult to accurately predict delayed release time, difficult to constant delayed release rate products, etc., to improve bioavailability, improve stability, and improve dissolution rate. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1-1
[0065] Preparation Example 1-1: Preparation of particles containing irbesartan
[0066] According to the composition described in Table 1, irbesartan (Hanmi Fine Chemical Co., Ltd., Korea), mannitol, pregelatinized starch and croscarmellose sodium (DMV international) were mixed, and then dissolved with A binding solution of povidone (BASF, Germany) in water knead the mixture, dry and pass through a 30-mesh sieve to obtain wet granules, then add magnesium stearate and mix to prepare Erbe Sartan granules.
preparation Embodiment 1-2
[0067] Preparation Example 1-2: Preparation of particles containing irbesartan
[0068] According to the composition described in Table 1, irbesartan (Hanmi Fine Chemical Co., Ltd., Korea), mannitol, pregelatinized starch and croscarmellose sodium (DMV international) were mixed, and then dissolved with A binding solution of povidone (BASF, Germany) and poloxamer 188 (BASF, Germany) in water knead the mixture, dry and pass through a 30-mesh sieve to obtain wet granules, and then add magnesium stearate And mixed to prepare irbesartan granules.
[0069]
[0070] Granules containing irbesartan (unit: mg)
[0071] ingredient Preparation Example 1-1 Preparation Example 1-2 Irbesartan 150 150 Mannitol 47 47 Pregelatinized starch 23 23 Croscarmellose Sodium 12 12 Povidone 8 8 Poloxamer 188 9
preparation Embodiment 2-1
[0072] Preparation Example 2-1: Preparation of particles containing atorvastatin
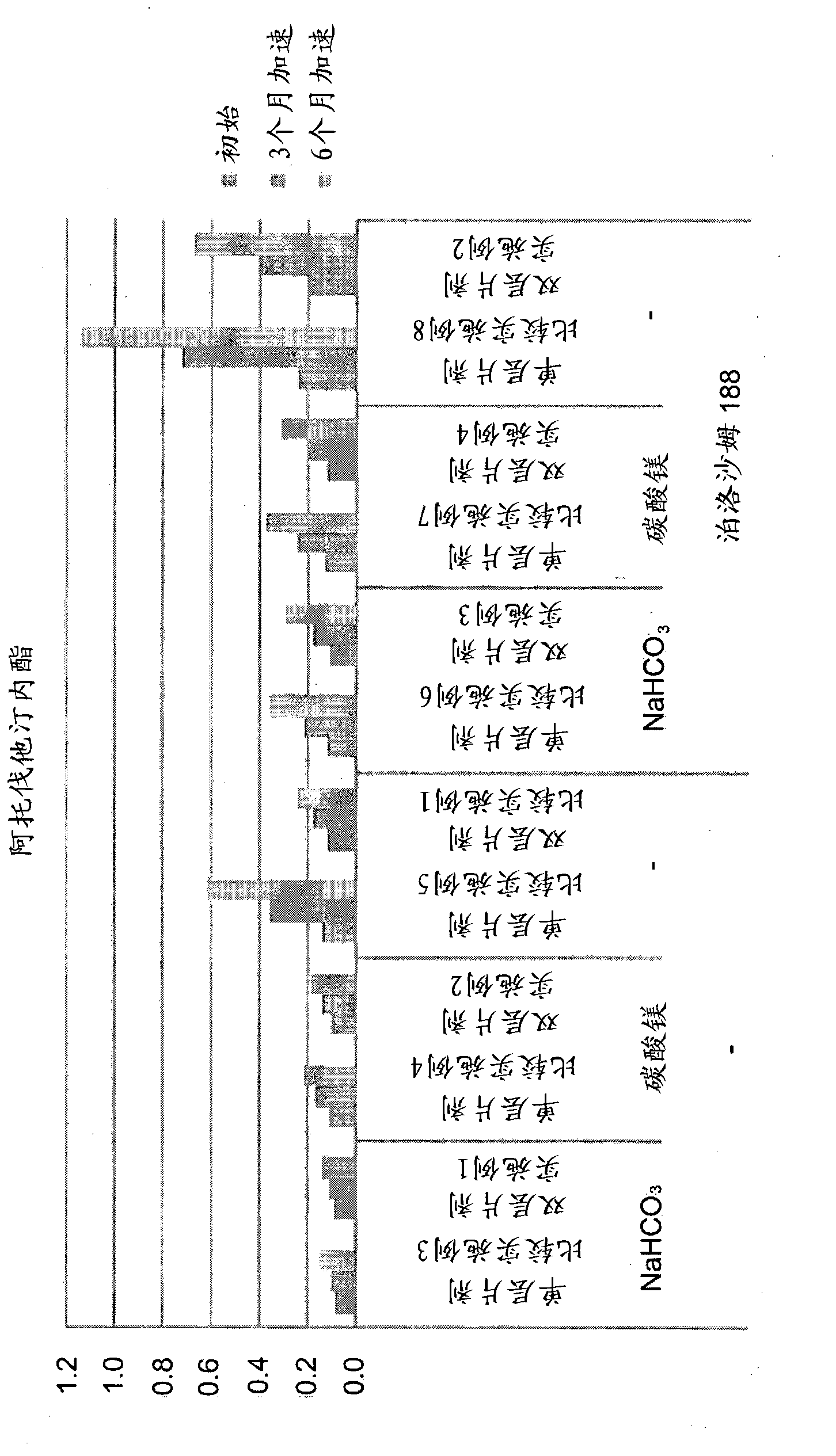
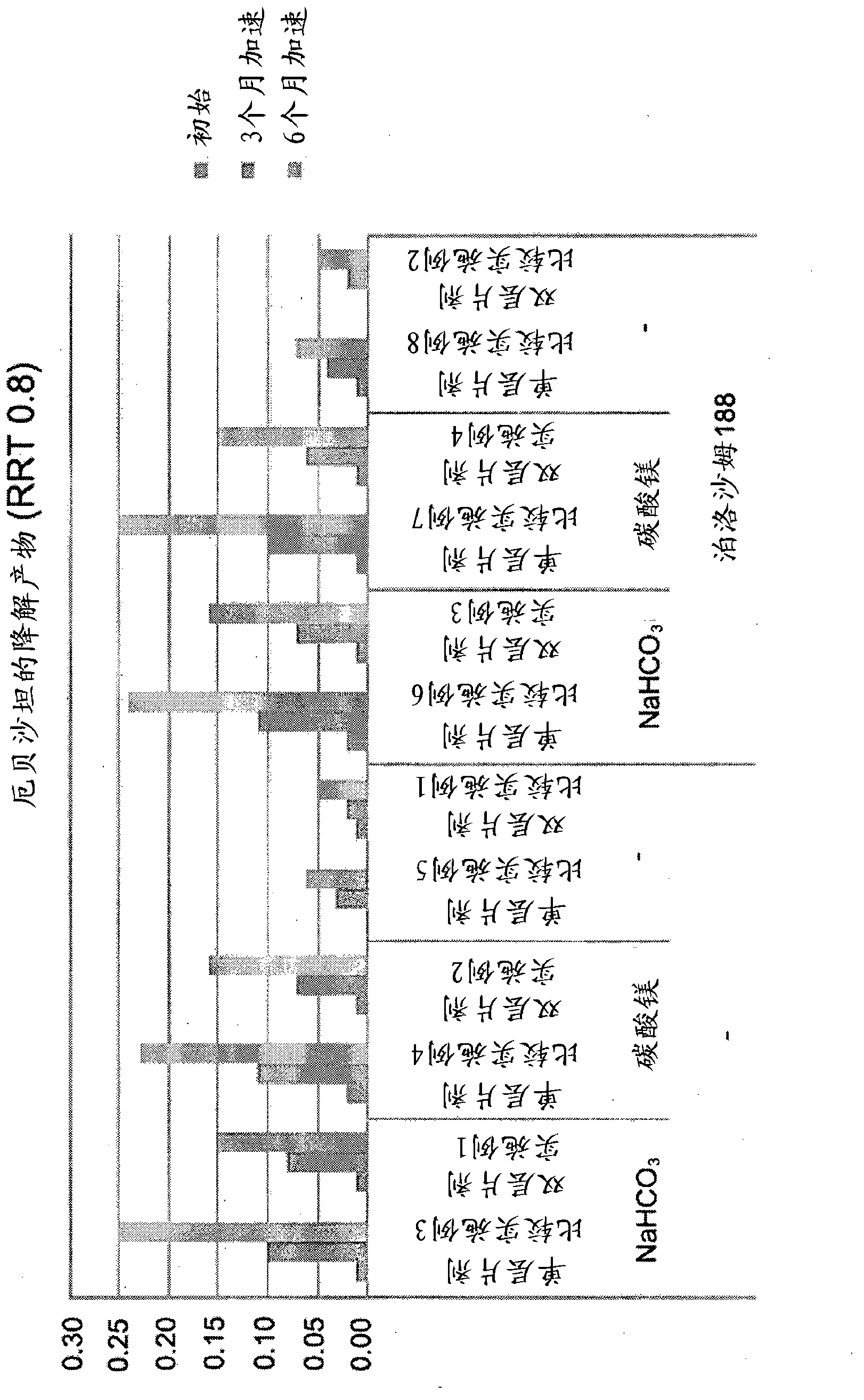
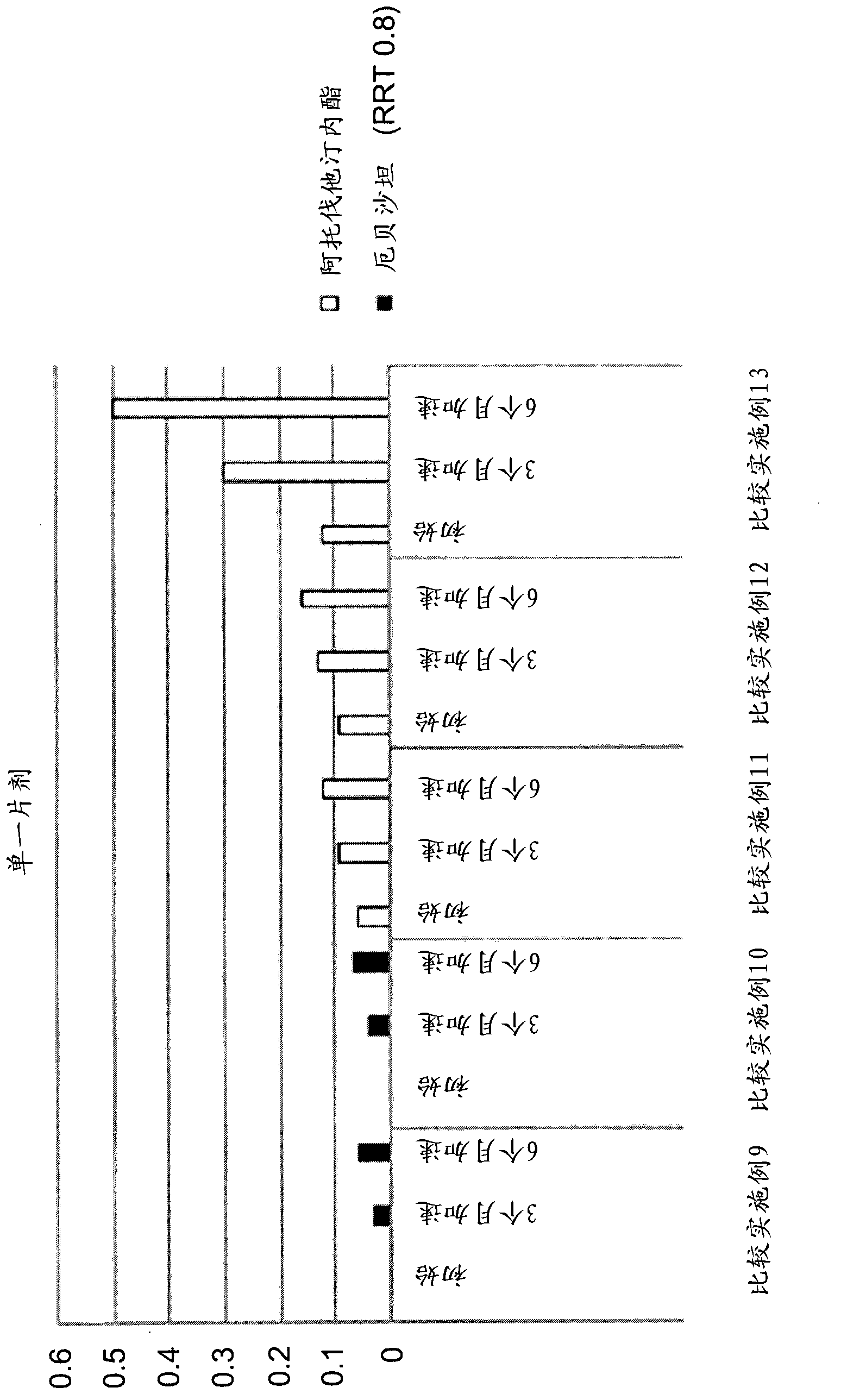
[0073] According to the composition described in Table 2, combine atorvastatin calcium (TEVA, India), mannitol, microcrystalline cellulose and crospovidone (BASF, Germany) with NaHCO 3 (Pendrice Soda, Australia), then knead the mixture with a binding solution of HPC (hydroxypropyl cellulose) and polysorbate 80 (Croda, USA) dissolved in water, dry and pass through a 30-mesh screen to Wet granules are obtained, and then magnesium stearate is added and mixed to prepare HMG-CoA reductase inhibitor granules.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com