Osteoclast culturing kit and preparation method and application thereof
A technology of osteoclasts and kits, applied in the fields of medicine and biology, can solve the problems of cumbersome operation steps, time-consuming and laborious, etc., and achieve the effects of high differentiation maturity, simplified operation steps, and strong absorption capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The preparation of embodiment 1 osteoclast culture kit
[0033] 1. Drugs and their sources
[0034] α-MEM culture medium (α-Minimal essential medium, product number: SH30265.01B), penicillin streptomycin (product number: SV30010), fetal bovine serum (fetal bovine serum (FBS) product number: SV30087.01) were purchased from Thermo Scientific HyClone Company (USA);
[0035] M-CSF (article number: 315-02) and RANKL (article number: 315-11) were purchased from peprotech company in the United States.
[0036] 2. Method
[0037] (1) Reagent A, the preparation of frozen bone marrow mononuclear macrophages
[0038] Aseptically remove the femur and tibia from the male C57BL / 6 mouse that has been killed by neck dislocation, peel off the soft tissue above it, subtract the two ends of the bone, and insert a 5ml syringe filled with α-MEM culture solution into the bone marrow cavity from one end of the bone , wash out the red bone marrow block in the cavity; blow the bone marrow c...
Embodiment 2
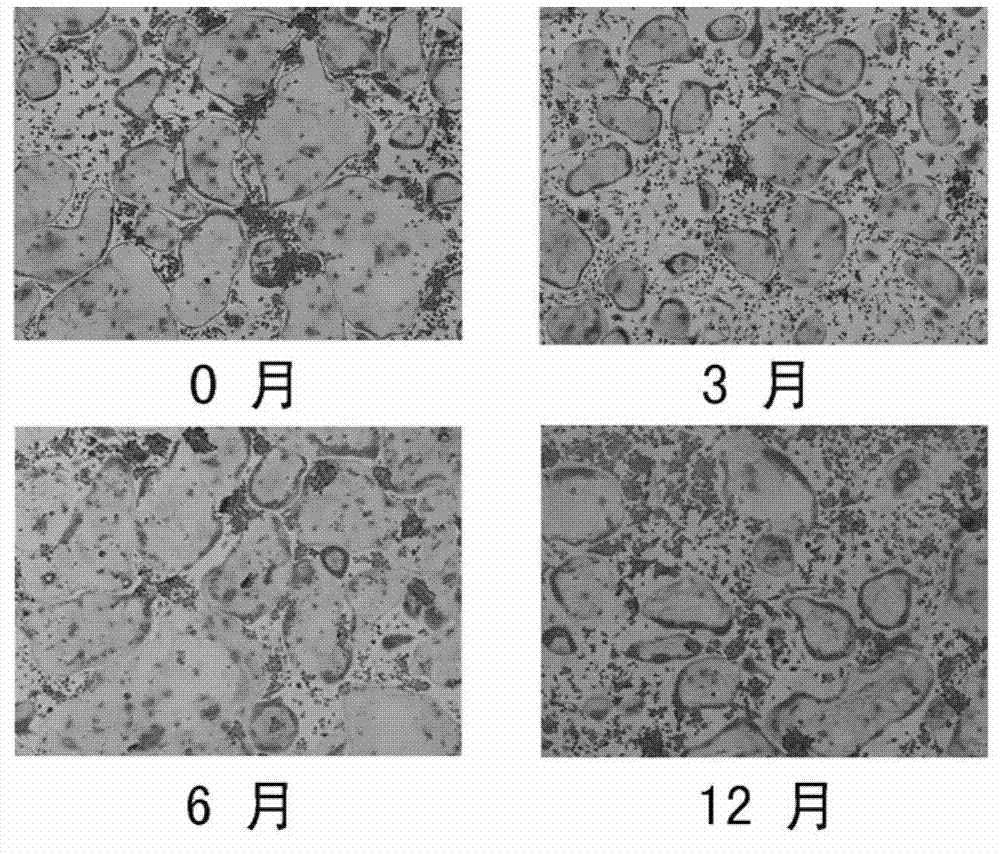
[0044] Example 2 Tartrate-resistant acid phosphatase (TRAP) staining experiment of osteoclasts cultured with the kit of the present invention
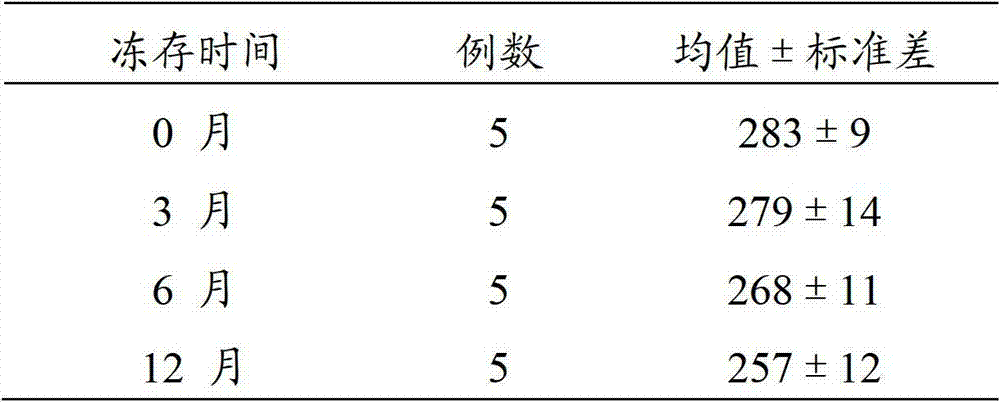
[0045] When reagent A (frozen-preserved bone marrow mononuclear macrophages) was frozen at -80°C for 0 months, 3 months, 6 months, and 12 months, place the cryopreservation tubes of reagent A in a water bath at 42°C, and from time to time Shake to make it thaw and thaw quickly, then take out the cells under sterile conditions, centrifuge at 1000 rpm for 5 minutes, discard the supernatant, add an appropriate amount of α-MEM culture medium, resuspend the cells, repeat the above steps once, to To achieve the purpose of removing the cell cryopreservation solution; the cells in the resuscitated reagent A, that is, bone marrow mononuclear macrophages, were mixed with 1×10 5 Inoculate each well in a 96-well plate, culture with reagent B for 3 days, replace the medium with reagent C, and use reagent C to change the medium every 3 days. On the...
Embodiment 3
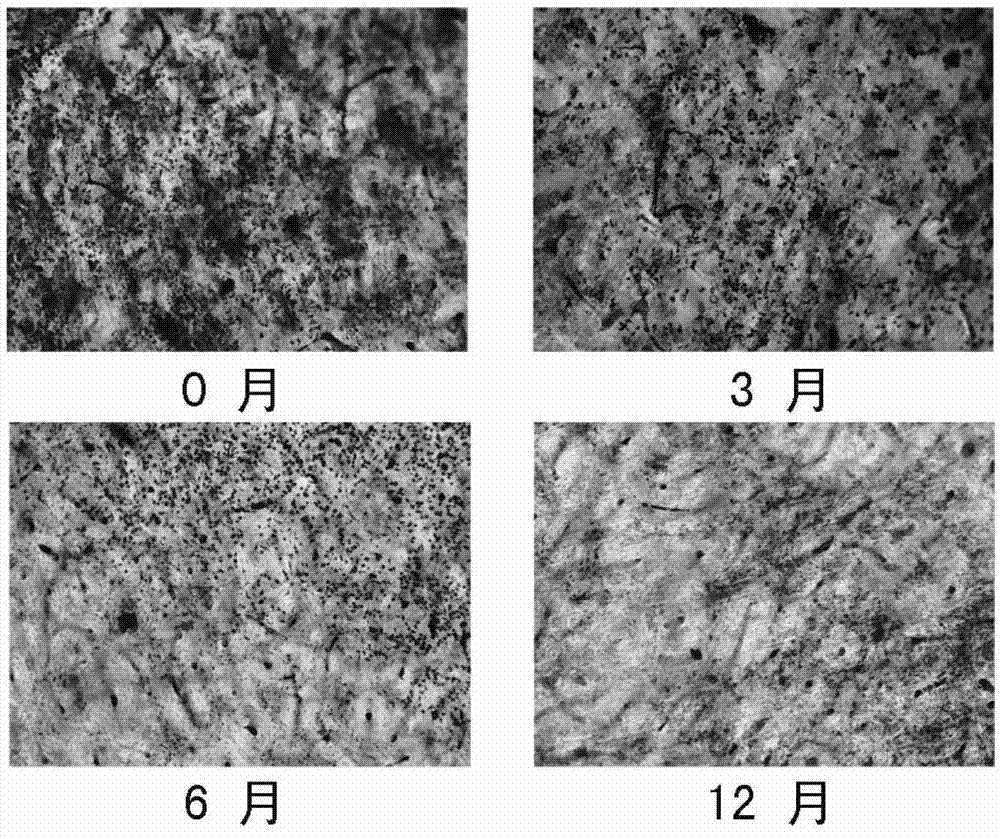
[0049] Embodiment 3 The bone resorption experiment of the osteoclast cultured with the kit of the present invention
[0050] Bovine bone slices with a diameter of 6 mm (purchased from IDS Company, UK) were placed in the wells of a 96-well culture plate, sterilized by ultraviolet irradiation in a ultra-clean bench for 1 hour, and the culture medium was washed 3 times. Place the cryopreservation tubes of Reagent A frozen at -80°C for 0 months, March, June, and December respectively in a 42°C water bath, and shake them from time to time to make them thaw quickly, and then take them out under sterile conditions Centrifuge the cells at 1000 rpm for 5 minutes, discard the supernatant, add an appropriate amount of α-MEM culture medium, resuspend the cells, and repeat the above steps once to achieve the purpose of removing the cell freezing solution; cells, that is, bone marrow mononuclear macrophages at 1×10 5 Inoculate each well in a 96-well plate, culture with reagent B for 3 days...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com