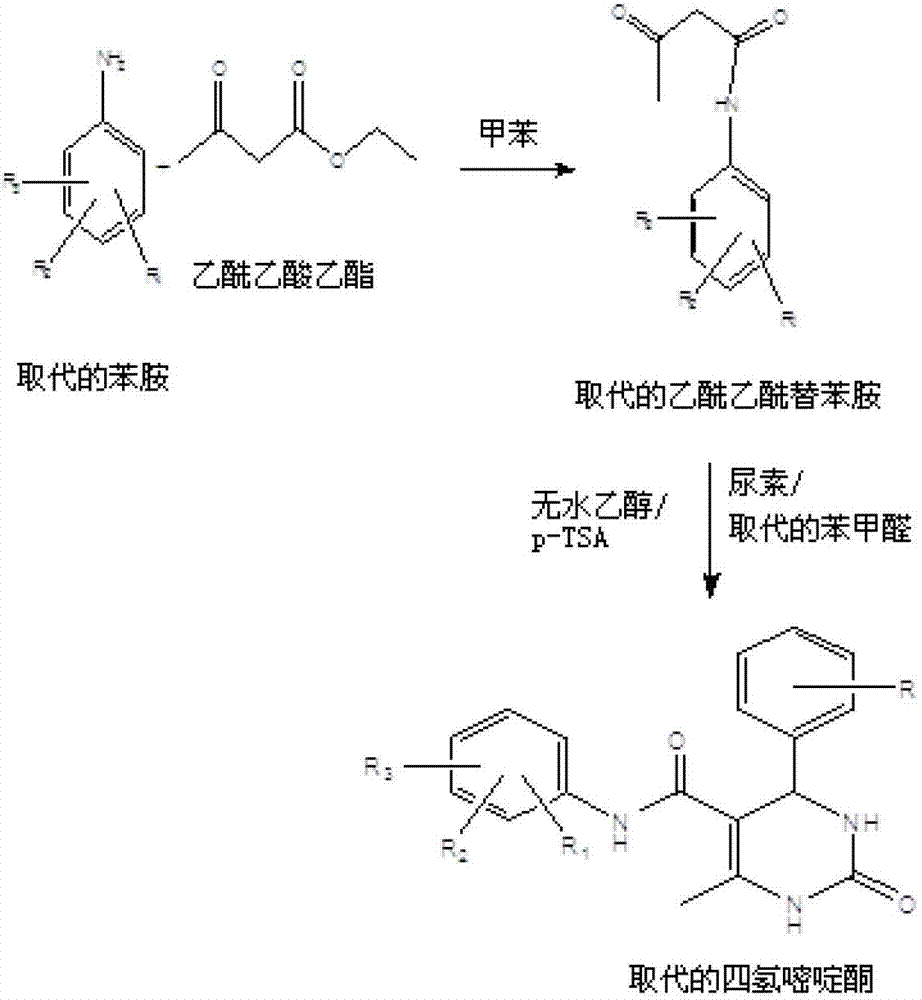
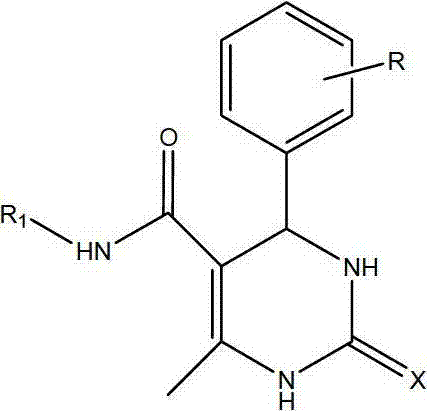
6 - methyl - 4 - phenyl - 5 - ( phenyl or cycloalkyl) carbamoyl - 1,2,3, 4 - tetrahydropyrimidin- 2 - one derivatives as antitubercular agents
A technology of ectoine and phenyl, applied in the field of preparing these compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0104] Example 1: N-(3-chlorophenyl)-4-(2,4-dichlorophenyl)-6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5 - amides. (1)
[0105] Synthesized using Experimental Procedure Method "A". (3-Chloroacetylacetylanilide, 2,4-dichlorobenzaldehyde)
[0106] Yield: 46%; melting point (m.p.) 201-202°C; infrared spectrum (IR) (KBr, cm-1): 3227, 2953, 1703, 1680, 1472, 1236, 772; 1 H NMR spectrum ( 1 H NMR) (400MHz, DMSO-d6) δppm 1.71 (s, 3H, CH3), 5.49 (s, 1H, CH) 7.02-7.53 (m, 7H, Ar-H), 7.6 (s, 1H, NH), 8.95 (s, 1H, NH) 9.45 (s, 1H, NH).
Embodiment 2
[0107] Example 2: N-(4-nitro-phenyl)-4-(2,4-dichloro-phenyl)-6-methyl-2-oxo-1,2,3,4-tetrahydro Pyrimidine-5-amide. (2)
[0108] Synthesized using Experimental Procedure Method "A". (3-Nitroacetylacetoanilide, 2,4-dichlorobenzaldehyde)
[0109] Yield: 55%; Melting point (m.p.) 225-227°C; Infrared spectrum (IR) (KBr, cm-1): 3375, 2924, 1693, 1545, 1237, 756; 1 H NMR spectrum ( 1 H NMR) (400MHz, DMSO-d6) δppm2.05 (s, 3H, CH3), 5.78 (s, 1H, CH), 7.42-8.17 (m, 7H, Ar-H), 7.69 (s, 1H, NH ), 9.03 (s, 1H, NH), 10.32 (s, 1H, NH).
Embodiment 3
[0110] Example 3: N-(2,3-dichloro-phenyl)-4-(2,4-dichloro-phenyl)-6-methyl-2-oxo-1,2,3,4- Tetrahydropyrimidine-5-amide. (3)
[0111] Synthesized using Experimental Procedure Method "A". (2,3-dichloroacetoacetanilide, 2,4-dichlorobenzaldehyde) yield: 60%; melting point (m.p.) 264-266°C; infrared spectrum (IR) (KBr, cm-1): 3286 ,2928,1707,1458,1214,765; 1 H NMR spectrum ( 1 H NMR) (400MHz, DMSO-d6) δppm 2.14 (s, 3H, CH3), 5.74 (s, 1H, CH), 7.36-7.70 (m, 6H, Ar-H), 7.46 (s, 1H, NH) , 8.95(s,1H,NH), 9.45(s,1H,NH).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com