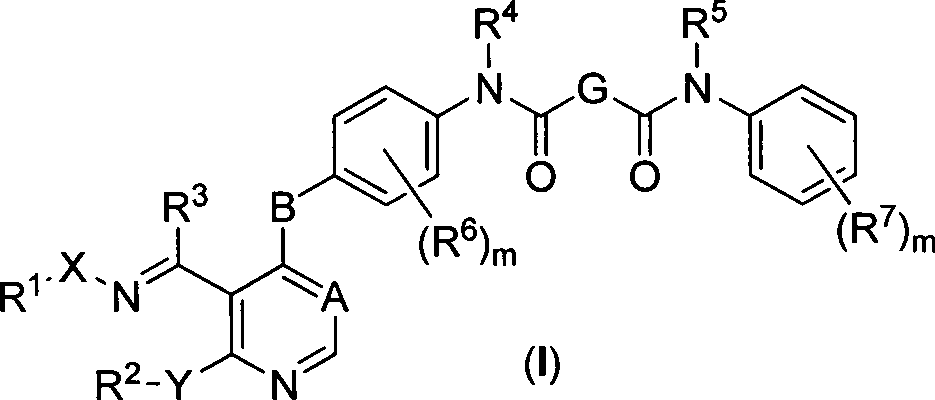
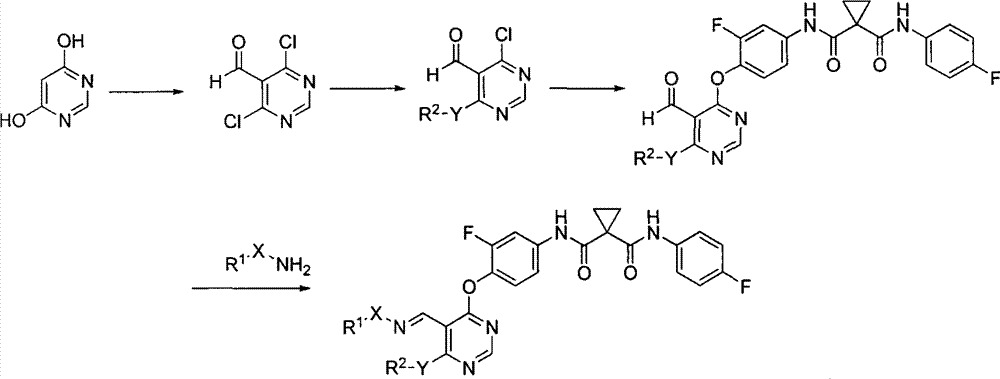
Novel pyrimidine compound, preparation method thereof, pharmaceutical composition containing novel pyrimidine compound and application of novel pyrimidine compound
A compound and hydrate technology, applied in the field of medicinal chemistry and pharmacotherapeutics, can solve the problem of low correlation of tumor incidence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0123] 4,6-Dichloropyrimidine-5-carbaldehyde (a)
[0124]
[0125] 4.8ml of DMF was added dropwise into 15ml of POCl3 which had been cooled in advance, and the internal temperature was controlled to be less than 10°C. After the dropwise addition was completed, it was stirred in an ice-water bath for 30 minutes. Add 3.75g of 4,6-dihydroxypyrimidine, transfer to an oil bath and heat to 100°C to react for 6h. After the reaction, the reaction solution was poured into crushed ice, extracted with ethyl acetate, and the organic phases were combined, washed with saturated aqueous sodium bicarbonate solution, water, and saturated brine, and dried over anhydrous sodium sulfate. After suction filtration, the filtrate was concentrated to obtain 2.75 g of an orange solid, with a yield of 69.6%.
[0126] 1 H NMR (300MHz, CDCl 3 )δ: 10.46 (S, 1H, -C H O), 8.90(s, 1H).
[0127]MS(ESI): 175.9[M+H] + .
Embodiment 2
[0129] 4-Amino-6-chloropyrimidine-5-carbaldehyde (b)
[0130]
[0131] Dissolve 15g of compound a in 300ml of toluene, slowly add 45ml of ammonia in methanol solution (4M) dropwise, and react at room temperature for about 20h. After the reaction, add water, extract with ethyl acetate (150ml×4), combine the organic phases, wash with water and saturated brine respectively, and dry over anhydrous sodium sulfate. After suction filtration, the filtrate was concentrated to obtain an orange solid, which was recrystallized by adding 100 ml of methanol to obtain 6.47 g of a light yellow solid with a yield of 48.5%.
[0132] 1 H NMR (300MHz, DMSO-d6) δ: 10.24 (S, 1H, -C H O), 8.74 (S, 1H, -N H ), 8.57 (S, 1H, -N H ), 8.40(s, 1H).
[0133] MS(ESI): 157.0[M+H] + .
Embodiment 3
[0135] N-[4-(6-amino-5-formylpyrimidine-4-oxygen)-3-fluorophenyl]-N-(4-fluorophenyl)cyclopropyl-1,1-dicarboxamide (c )
[0136]
[0137] 3.2g compound b was dissolved in 60ml DMF, added 7.61g K 2 CO 3 And 6.13g of N-(3-fluoro-4-hydroxyphenyl)-N-(4-fluorophenyl)cyclopropyl-1,1-dicarboxamide, stirred at room temperature for 2h. After the reaction, add water, extract with ethyl acetate (50ml×4), combine the organic phases, wash with water and saturated brine respectively, and dry over anhydrous sodium sulfate. After suction filtration, the filtrate was concentrated to obtain a yellow-white solid, and 50 ml of methanol was added, stirred and filtered to obtain 5.88 g of off-white solid, with a yield of 70.2%.
[0138] 1 H NMR (300MHz, DMSO-d6) δ: 10.37(s, 1H, -C H O), 10.32(s, 1H, -CON H -), 10.03(s, 1H, -CON H -), 8.53(s, -N H 2 , 2H), 8.18(s, 1H), 7.81-7.76(dd, J 1 =1.89,J 2 =12.84, 1H), 7.65-7.61 (m, 2H), 7.43-7.40 (m, 1H), 7.36-7.30 (t, J=8.71, 1H), 7.18-7.12 (t,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com