Method for catalyzing H2O2 processing of phenol in waste water by Schwertmannite
A technology of H2O2 and phenol, applied in chemical instruments and methods, oxidized water/sewage treatment, water/sewage treatment, etc., can solve the problems of difficult separation and reuse of catalysts, increased difficulty and cost, low reaction pH value, etc., to achieve The effect of rapid and thorough removal, easy management and control, simple and safe operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
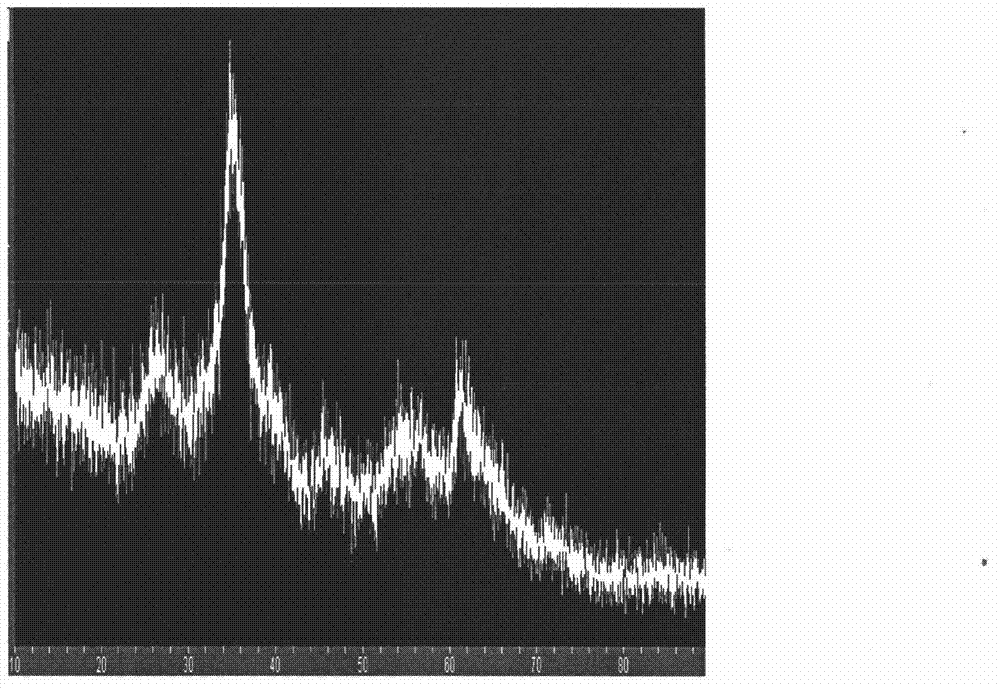
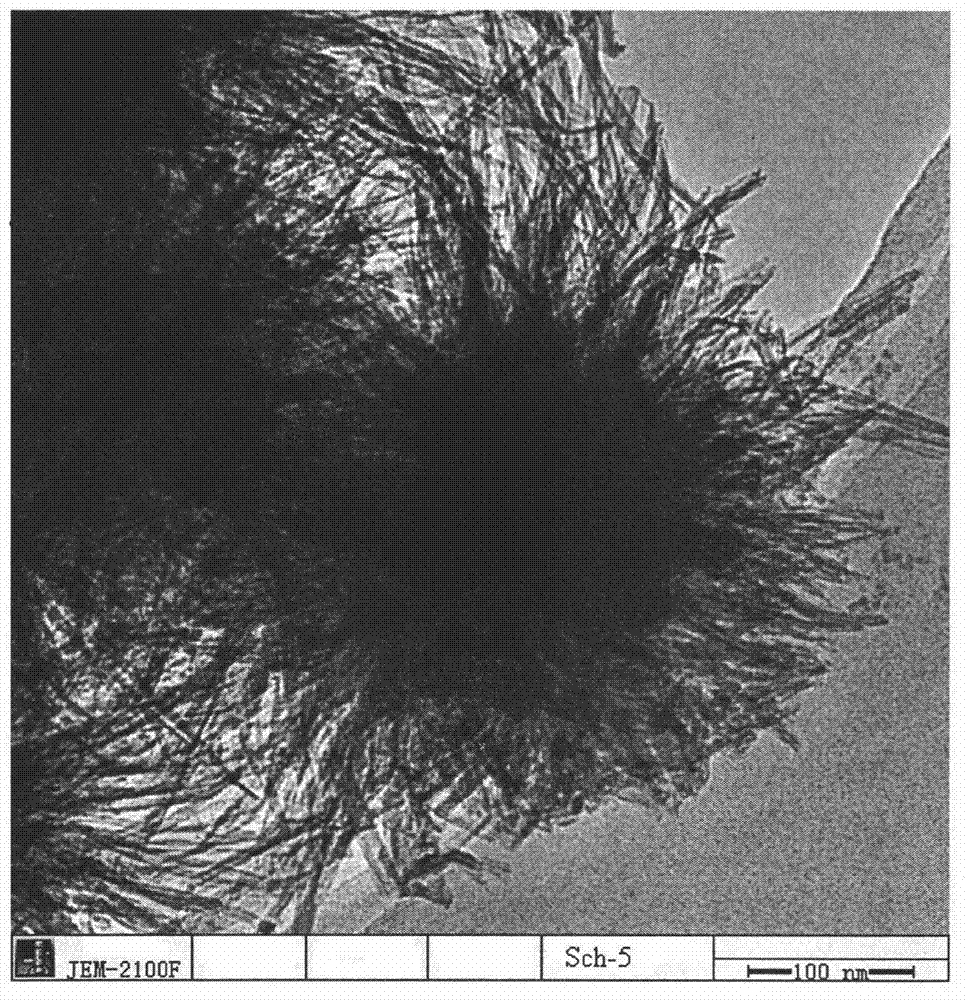
[0027] Add 500mL distilled water into the round bottom flask, preheat it in 85℃ water bath, then weigh 2.6g Fe 2 (SO 4 ) 3 , added to preheated water, and stirred vigorously at constant temperature for 1 hour; then stopped stirring, cooled naturally, washed the solid three times with distilled water, and freeze-dried. The morphology of the obtained product is shown in Figure 1.
Embodiment example 2
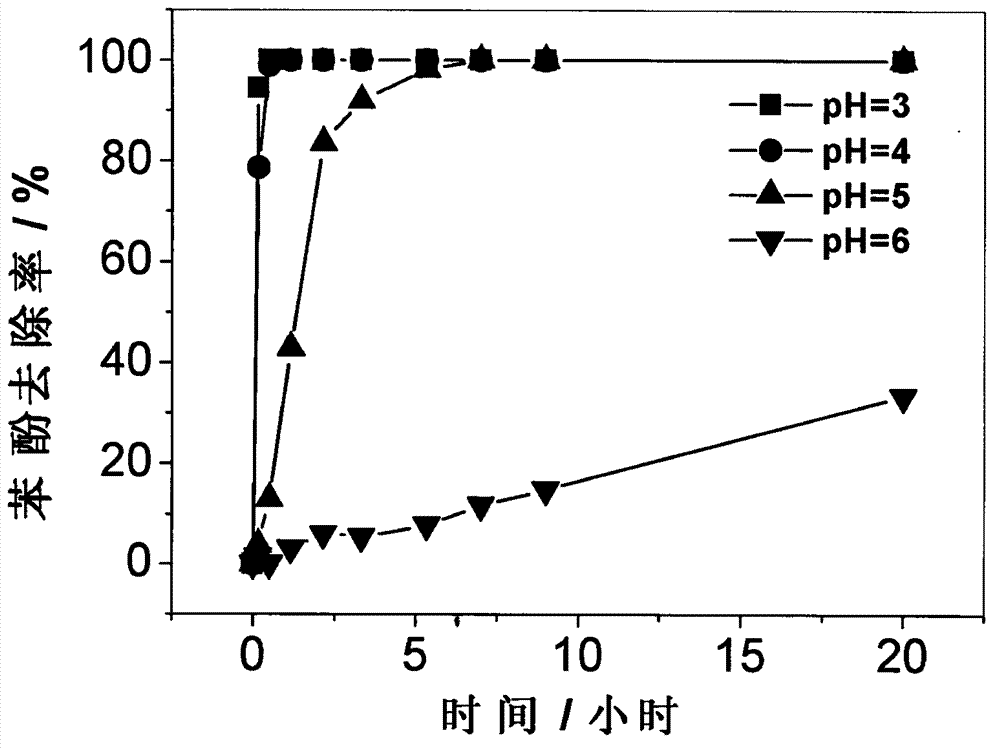
[0029] Mix the Shi-type mineral and phenol solution into a 250mL Erlenmeyer flask, wherein the Shi-type mineral and phenol concentrations are 0.25g / L-2g / L and 100mg / L, and then add H 2 o 2 , so that the concentration is 100mg / L-2000mg / L, adjust the pH value to 2-7, and shake the reaction at 145rpm on a shaker. attached by figure 2 It is known that Shi-type minerals have similar catalytic efficiency at pH = 5 and pH = 3 and pH = 4 in a weakly acidic environment. After 5 hours of oxidation reaction, the removal rate of phenol can reach 100%. attached by image 3 know, when the pH is 5, H 2 o 2 When the concentration is 500mg / L, 1g / L Shi-type mineral has higher catalytic efficiency, which is slightly lower than 2g / L Shi-type mineral. After 5 hours of oxidation reaction, the removal rate of phenol can reach 100%. attached by Figure 4 It can be seen that when the pH is 5 and the concentration of Shi formula minerals is 1g / L, 500mg / L H 2 o 2 With 1000mg / L and 2000mg / L H 2 ...
Embodiment example 3
[0031]Take 0.15g of Shishi minerals and add them to a 250mL Erlenmeyer flask, then add 100mL of 0.1M sodium chloride solution, adjust the pH value to 5, and stabilize it for 2 hours; another appropriate amount of 1g / L phenol solution is mixed with 0-0.5M chlorine A total of 50 mL of sodium chloride solution, 0-0.5M sodium sulfate solution, and 0-0.5M sodium nitrate solution were mixed into another 250 mL conical flask, and the pH value was adjusted to 5, and then the solutions in the two conical flasks were mixed. The concentration of phenol in the solution is 100mg / L, after fine-tuning the pH value, quickly add H 2 o 2 , so that the concentration is 500mg / L, shake the reaction at 145rpm on a shaker. It can be seen from accompanying drawing 5 that chloride ions, sulfate ions and nitrate ions have no obvious influence on the oxidation process, so this process can be adapted to the oxidation treatment of phenol in a certain concentration or even high concentration saline indust...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com