Eubacterium biforme preparation and use thereof
A technology of Eubacterium and preparation, applied in the field of biological medicine, can solve the problem that the curative effect is not very ideal and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0039] Preparation of Medicine Example 1 Preparation of Oral Protruding Eubacterium Live Bacteria Powder, Oral Dimorph Bacteria Live Bacteria Powder and Dual Live Bacteria Powder
[0040] 1 Preparation of bacteria powder and identification of strains
[0041] The feces of a healthy baby in Jiaonan City, Shandong Province were collected, and then the feces were placed in a sterilized anaerobic bottle, and nitrogen gas was blown in while fully mixing, and 2 grams of feces were quickly added to 18 mL of sterilized diluent, and nitrogen gas was blown in while fully mixing. Evenly, in the aseptic operating table, carry out 10 -1 、10 -2 、10 -3 、10 -4 、10 -5 、10 -6 、10 -7 Gradient dilution, take 10 -5 、10 -6 、10 -7 Three dilution gradients, spread on the solid medium for the selective single colony isolation of Eubacterium protrudoides or Eubacterium dimorphus, place in an anaerobic tank, culture at 37°C for 48 hours in anaerobic conditions, and select two single colonies wi...
Embodiment 1
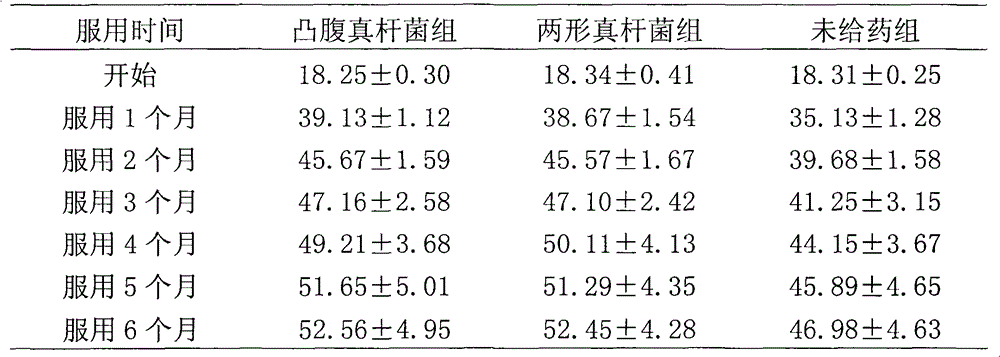
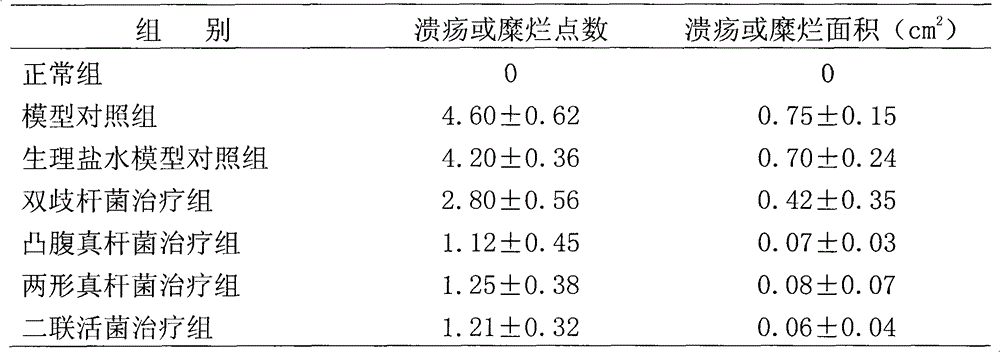
[0056] Example 1. Research on the repairing effect of Eubacterium protrudoides and Eubacterium dimorphus on intestinal mucosa
[0057] 1 Materials and methods
[0058] 1.1 Materials
[0059] 1.1.1 drug protruding fungus liquid (10 7 CFU / mL); Amphimorpha bacterial liquid (10 7 CFU / mL); dual live bacteria solution (10 7 CFU / mL); Bifidobacteria liquid (10 7 CFU / mL); dextran sodium sulfate (DSS, 10g / bottle, Sino-American Biotec); Alcian blue (1g / bottle, Sino-American Biotec).
[0060] 1.1.2 Animals 70 male SD rats, weighing 80-100 g, standard grade 2, were purchased from the Experimental Animal Center of China Institute for the Control of Pharmaceutical and Biological Products.
[0061] 1.2 Method
[0062] 1.2.1 Modeling and treatment of intestinal mucosal injury
[0063] There were 70 rats, 10 rats were used as the normal control group, and the other 60 rats were fed with 3% DSS solution (1mL / 100g) to the fasting rats, once a day for 7 days, and the last time the anal inje...
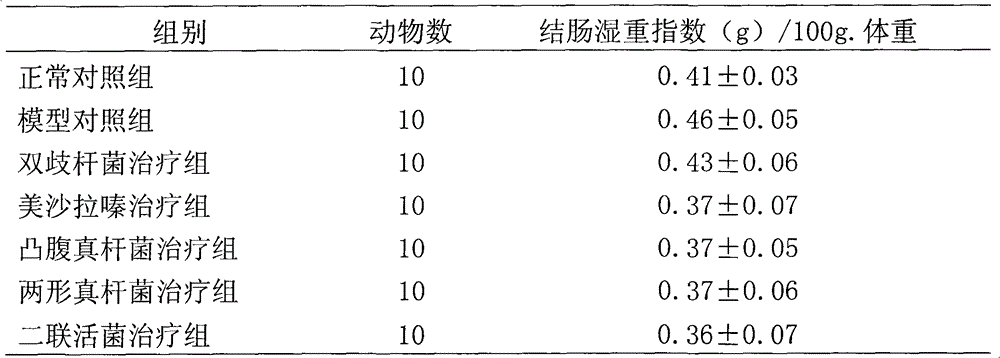
Embodiment 2
[0072] Example 2. Studies on the Regulatory Expression of Immunity and Inflammatory Factors by Eubacterium protrudoides and Eubacterium amphimorpha
[0073] 1 Experimental materials and methods
[0074] 1.1 Experimental animals: male SD rats, 180-220g.
[0075] 1.2 Experimental drug: mesalamine (200mg / mL); protruding fungus liquid (10 7 CFU / mL); Amphimorpha bacterial liquid (10 7 CFU / mL); dual live bacteria solution (10 7 CFU / mL); Bifidobacteria liquid (10 7 CFU / mL). Provided by Qingdao Donghai Pharmaceutical Co., Ltd.
[0076] 1.3 Experimental reagents: Freund's complete adjuvant (sigma), MTT (sigma); concanavalin (ConA) (Sigma); lipopolysaccharide (LPS) (Sigma); MTT (Sigma), purchased from Beijing Shubowei Chemical Industry Instrument Co., Ltd.; 1640 culture medium (Gibco); lyophilized powder of bovine colonic mucosal protein (self-made); IL-8 ELISA kit (BD); TNF-α ELISA kit (Ebioscience); rat IgG reference serum; Mouse IgG antiserum was purchased.
[0077] 2 Experim...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Average age | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com