Acrylyl lysine translation system and application thereof
A technology of acryloyl lysine and acryloyl lysine aminoacyl, applied in the field of biochemistry, can solve the problem that inducibility is not widely understood
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
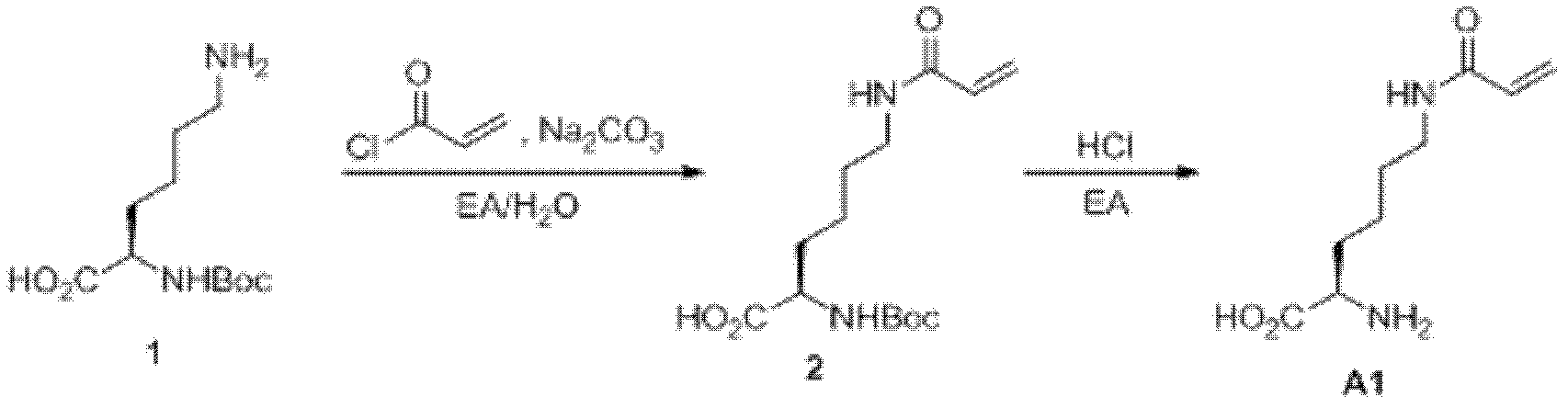
[0045] Embodiment 1: chemical synthesis
[0046] 1. Synthesis of acryloyllysine (AcrK) ( figure 1 ): Add compound N-α-Boc-lysine (2.46g, 10.0mmol, purchased from Shanghai Gil Biochemical Company) and anhydrous sodium carbonate (2.12g, 20.0mmol), and then add 100mL of ethyl acetate and water mixed solvent (ethyl acetate: water (v / v) = 1: 1), slowly add 1.1 equivalents of acryloyl chloride (purchased from TCI company) under stirring in an ice bath ethyl acetate solution. React overnight, adjust the pH to 3 with glacial acetic acid, extract with ethyl acetate, add 100 mL of ethyl acetate hydrogen chloride solution under ice bath after rotary evaporation, stir overnight, a large amount of white solid precipitates, filter, and wash the solid several times with ethyl acetate , the target compound acryloyllysine (1.44 g, 7.2 mmol) was obtained after drying, and the yield was 72%. 1 H NMR (600MHz, D 2 O) δ=1.38-1.47 (m, 2H), 1.55-1.59 (m, 2H), 1.89-1.97 (m, 2H), 3.26 (dd, J 1 =6....
Embodiment 2
[0051] Example 2: Expression of AcrK-green fluorescent protein (GFP) and identification by mass spectrometry
[0052] In order to site-specifically incorporate acryloyllysine (AcrK) into the gene, it is necessary to introduce an acryloyllysine aminoacyl tRNA synthetase / tRNA orthogonal pair into the E.coli host cell used, this orthogonal pair Amber suppressor tRNA (Mb tRNA) from Methanosarcina barkeri CUA Pyl ) / aminoacyl tRNA synthetase (Mb PylRS) pair. Orthogonal tRNA (SEQ ID NO: 1) and acryloyllysine aminoacyl tRNA synthetase mutant (SEQ ID NO: 2) (Heinz Neumann, Sew Y Peak-Chew, Jason W Chin, Genetically encoding Ne-acetyllysine in recombinant proteins, Nature chemical biology, 4, 4, 2008; Neumann H, Hancock SM, Buning R, et al, Mol Cell, 2009 Oct9; 36(1): 153-63) were constructed into the pEVOL vector (US scripps research Peter G. Schultz Laboratory), and then co-transformed into DH10B cells (purchased from Quanshijin Company) containing pEt22b-GFP-151TAG (SEQ ID NO: 4) ...
Embodiment 3
[0054] Example 3: Expressing AcrK-Ftsz and performing in vivo and in vitro photoclick reactions
[0055] The wild-type Ftsz (SEQ ID NO: 6, derived from Escherichia coli BL21) was constructed on the pEt22b vector (purchased from Novagen), and the orthogonal tRNA (SEQ ID NO: 1) and acryloyllysine aminoacyl tRNA The nucleotide sequences (SEQ ID NO: 3) of the synthetase mutants were respectively constructed on the pEVOL vector (gifted by the laboratory of Peter G. Schultz, Scripps Research Institute, USA). A TAG (SEQ ID NO: 8, Ftsz-3TAG, wherein the amino acid at the 3rd position is acryloyl lysine, represented by @) was introduced at the 3rd position of Ftsz by PCR method. Cotransform pEVOL-tRNA (that is, a recombinant vector comprising an orthogonal tRNA sequence (SEQ ID NO: 1)), pEVOL-AcrKRS (that is, a nucleotide sequence comprising an acryloyllysine aminoacyl tRNA synthetase mutant (SEQ ID NO: 1) ID NO: 3) recombinant vector) and pEt22b-Ftsz-3TAG into BL21 (DE3) cells (purch...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com