Riemerella anatipestifer tervalent inactivated vaccine and preparation method thereof
A technology of Riemerella anatipestifer and inactivated vaccines, which is applied in the field of trivalent inactivated vaccines of Riemerella anatipestifer and its preparation, can solve the problem of no preventive protection, no research reports, and difficulty in reaching the trivalent number of bacteria Requirements for the number of bacteria in inactivated vaccines and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0026] Below in conjunction with specific embodiment, further illustrate the present invention. These examples are only for illustrating the present invention and are not intended to limit the scope of the present invention. For the experimental methods without specific conditions indicated in the following examples, usually follow the conventional conditions or the conditions suggested by the manufacturer. Unless otherwise defined, all professional and scientific terms used herein have the same meanings as commonly understood by those skilled in the art.
[0027] The reagents and raw materials not marked in the present invention are purchased from the market.
[0028] 1: Rejuvenation and identification of candidate strains for seedling production
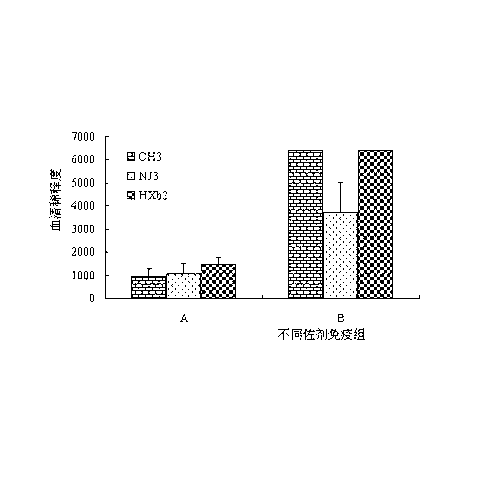
[0029] Our previous research obtained candidate strains CH3, NJ3 and HXb2 for serotype 1, 2 and 10 vaccine production. The morphology, biochemical reaction characteristics, molecular biological characteristics and immune protect...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com