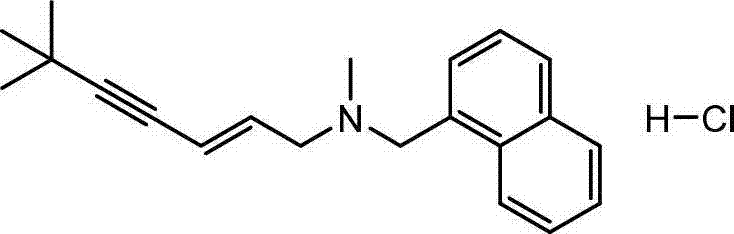
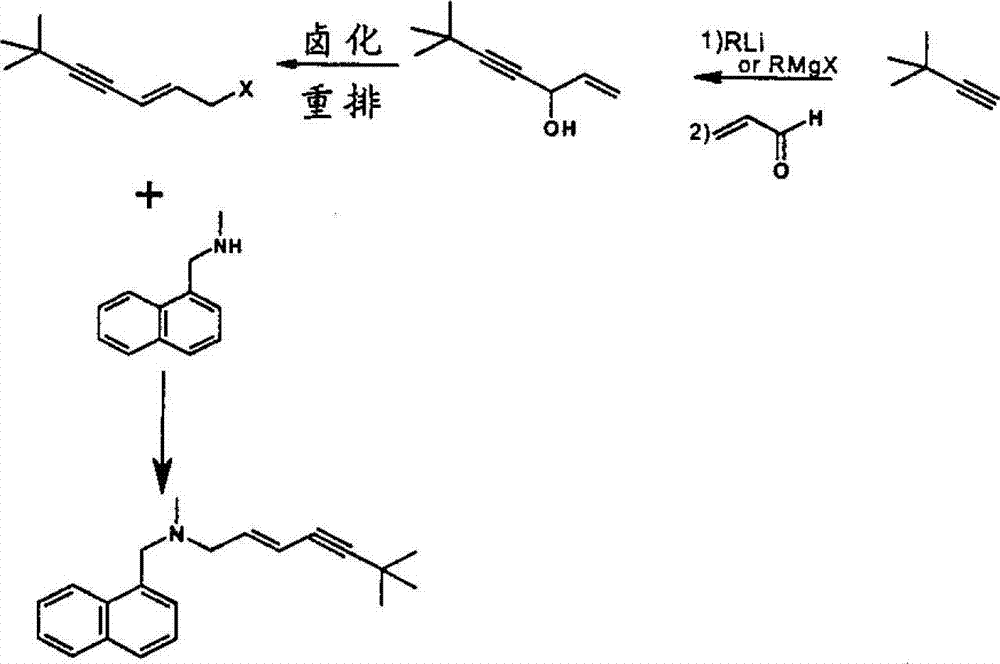
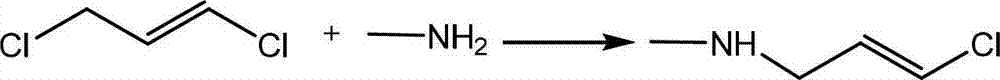Preparation method of terbinafine hydrochloride
A technology of terbinafine hydrochloride and monomethylamine, applied in the field of medicine, can solve the problems of unsuitability for industrialized production, high production cost, rare raw materials, etc., and achieve the effects of simple production steps, low price, and easily available raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Step 1: Synthesis of (E)-1-methylamino-3-chloro-propene
[0032] Add 200 grams of monomethylamine aqueous solution (30%) into a 500ml reaction bottle, start stirring, cool down to 0°C, add 0.5 grams of tributylammonium bromide, and drop 50 grams of (E)-1,3 dichloropropene , slowly raised to 20°C, reacted for 4 hours, monitored (E)-1-methylamino-3-chloro-propene remaining 3%, continued to rise to 25°C for 2 hours, remained 1.3%, separated. The product was in the lower layer, and the residual monomethylamine was washed with water, then 5 g of sodium sulfate was added as a desiccant, dried, and 45 g of the product was obtained by suction filtration, with a content of 97.5% and a yield of 92.5%. For this step reaction, the sealing performance of the reaction vessel is required to be better.
[0033] Step 2: Synthesis of (E)-N-(6,6-dimethyl-2-hepten-4-ynyl)methanamine
[0034] Put 45 grams of (E)-1-methylamino-3-chloro-propene in step 1 into the reaction flask, add 100 gra...
Embodiment 2
[0038] Step 1: Synthesis of (E)-1-methylamino-3-chloro-propene
[0039]Add 380 grams of monomethylamine aqueous solution (30%) into a 1000ml reaction bottle, start stirring, cool down to 2°C, add 1.0 grams of tributylammonium bromide, add dropwise 100 grams of (E)-1,3 dichloropropene , slowly raised to 20°C, reacted for 3 hours, monitored (E)-1-methylamino-3-chloro-propene remaining 3.8%, continued to rise to 25°C for 2.5 hours, remained 1.2%, separated, the product was in the lower layer , washed the residual monomethylamine with water, then added 10 grams of desiccant sodium sulfate, dried, and suction filtered to obtain 90.5 grams of the product, with a content of 97.3% and a yield of 92.7%. For this step reaction, the sealing performance of the reaction vessel is required to be better.
[0040] Step 2: Synthesis of (E)-N-(6,6-dimethyl-2-hepten-4-ynyl)methanamine
[0041] Put 90.5 grams of (E)-1-methylamino-3-chloro-propene in step 1 into the reaction flask, add 190 grams...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com