Anti-tetanotoxin antibody, and preparation method and application thereof
An anti-tetanus and antibody technology, applied in the direction of botany equipment and methods, biochemical equipment and methods, applications, etc., can solve the problems of no practical application value, side effects and large acceptance by blood donors, and achieve high affinity and preparation The method is simple and the application prospect is broad
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
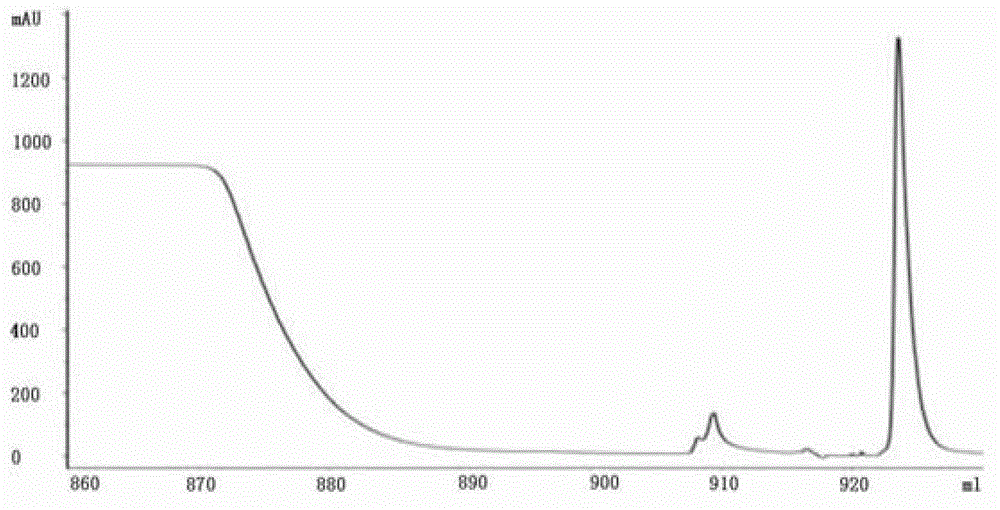
Embodiment 1
[0031] Embodiment 1 Preparation of antibody of the present invention
[0032] 1. Human immune antibody library construction
[0033] The peripheral blood of 5 healthy adults with high neutralization titer immunized with purified tetanus toxoid was collected by conventional method, and the mononuclear cells were separated;
[0034] Total RNA was extracted with Trizol, and single-stranded cDNA was synthesized by reverse transcription according to the M-Mulv-RT reagent instructions, and used as a template to PCR amplify the light and heavy chain genes of different families with the following primers;
[0035] KI-5'ACCGGAGAGCTCCAGATGACCCAGTCTCCA.
[0036] KII-5'ACCGGAGAGCTCGTGWTGACYCAGTCTCCA.
[0037] The above two primers are primers at the 5' end of the light chain, and the underline is the SacI restriction site.
[0038] K-3'GCGCCGTCTAGAACTAACACTTCCCCCCTGTTGAAGCTCTTTGTGACGGGCAAG.
[0039] The above primers are primers for the 3' end of the light chain, and the underline i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com